Abstract
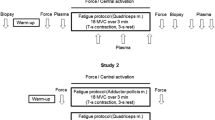
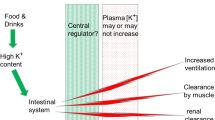
To examine the simultaneous changes in plasma [K+], muscle excitability and force during fatigue, ten male adults (mean age = 22 ± 0.5 years) held an isometric contraction of their right quadriceps muscle at an intensity of 30% maximum voluntary contraction (MVC) for 3 min. Femoral venous and brachial arterial [K+] were determined from serial samples drawn before, during, and for 15 min following the 3-min contraction. Each blood sample was synchronized with a maximal stimulation of the right femoral nerve to evoke a twitch and compound muscle action potential (M-wave). Immediately post-exercise, twitch torque was only 42% of baseline and femoral venous plasma [K+] had increased significantly from 4.02 ± 0.08 mmol/l to 5.9 ± 0.22 mmol/1. Femoral venous plasma lactate rose to a peak level of 10.0 ± 0.8 mmol/1 at 1 min post exercise. The recovery of the twitch torque was exponentially related to the recovery of femoral venous plasma [K+] (r2 = 0.93, P < 0.01). There was no evidence for any loss of muscle membrane excitability during the period of increased extracellular [K+], in fact, the M-waves tended to be potentiated in the early phases of the recovery period. These results suggest that muscle membrane excitability is maintained in spite of increased extracellular [K+] following fatigue induced by a sustained submaximal quadriceps contraction. However, the strong relationship between twitch torque and femoral venous plasma [K+] suggests that K+ may be exerting its effect distal to surface membrane action potential propagation, most likely in the T-tubular region.
Similar content being viewed by others
References
Ashley C, Ridgway EB (1970) On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol (Lond) 209:105–130
Belanger AY, McComas AJ (1981) Extent of motor unit activation during effort. J Appl Physiol 51:1131–1135
Bellemare F, Garzaniti N (1988) Failure of neuromuscular propagation during human maximal voluntary contraction. J Appl Physiol 64:1084–1093
Bigland-Ritchie B, Woods JJ (1984) Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 7:691–699
Bigland-Ritchie B, Jones DA, Woods JJ (1979) Excitation frequency and muscle fatigue: Electrical responses during human voluntary and stimulated contractions. Exp Neurol 64: 414–427
Bigland-Ritchie B, Kukulka CG, Lippold OCJ, Woods JJ (1982) The absence of neuromuscular transmission failure in sustained maximal voluntary contractions. J Physiol (Lond) 330:265–278
Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OCJ (1986) Reflex origin for the slowing of motoneuron firing rates in fatigue of human voluntary contractions. J Physiol (Lond) 379:451–459
Clausen T (1986) Regulation of active sodium/potassium transport in skeletal muscle. Physiol Rev 66:542–580
Clausen T, Everts ME (1989) Regulation of the Na+/K+ pump in skeletal muscle. Kidney Int 35:1–13
Fuglevand AJ, Zackowski KM, Huey KA, Enoka RM (1993) Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol (Lond) 460:549–572
Garland SJ, McComas AJ (1990) Reflex inhibition of human soleus muscle during fatigue. J Physiol (Lond) 429:17–27
Garland SJ, Enoka RM, Serrano LP, Robinson GA (1994) Behaviour of motor units in human biceps brachii muscle during a submaximal fatiguing contraction. J Appl Physiol 76: 2411–2419
Hicks A, McComas AJ (1989) Increased sodium pump activity following the stimulation of rat soleus muscles. J Physiol (Lond) 414:337–349
Hicks A, Fenton J, Garner S, McComas AJ (1989) M-wave potentiation during and after muscle activity. J Appl Physiol 66:2606–2610
Hirche HE, Schumacher E, Hagemann H (1980) Extracellular potassium concentration and potassium balance of the gastrocnemius muscle of the dog during exercise. Pflügers Arch 387:231–237
Hnik P, Vyskocil F, Ujec E, Vejsada R, Rehfeldt H (1986) Work induced potassium loss from skeletal muscles and its physiological implications. Int Ser Sports Sci 16:345–364
Hodgkin AL, Katz B (1949) The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol (Lond) 108:37–77
Hodgkin AL, Huxley AF (1952) The components of membrane conductance in the giant axon of Loligo. J Physiol (Lond) 116: 473–496
Juel C (1986) Potassium and sodium shifts during in vitro isometric muscle contraction, and the time course of the ion-gradient recorvery. Pflügers Arch 406:458 463
Kjaer M (1989) Epinephrine and some other hormonal responses to exercise in man: With specific reference to physical training. Int J Sports Med 10:2–15
Lind AR, Petrofsky JS (1979) Amplitude of the surface electromyogram during fatiguing isometric contractions. Muscle Nerve 2:257–264
Lindinger MI, Heigenhauser GJF (1988) Ion fluxes during tetanic stimulation in isolated perfused rat hindlimb. Am J Physiol 254:R117-R126
Lindinger MI, Sjøgaard G (1991) Potassium regulation during exercise and recovery. Sports Med 11:382–401
Maton B (1991) Central nervous changes in fatigue induced by local work. In: Atlan G, Beliveau L, Boissou P (eds) Muscle fatigue: biochemical and physiological aspects. Masson, Paris, pp 207–221
McKenna MJ (1992) The roles of ionic processes in muscular fatigue during intense exercise. Sports Med 13:134–145
Medbo JI, Sejersted OM (1990) Plasma potassium changes with high intensity exercise. J Physiol (Lond) 421:105–122
Person RS, Kudina LP (1972) Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol 32: 471–483
Petrofsky JS, Phillips CA (1986) The physiology of static exercise. Exerc Sport Sci Rev 14:1–44
Renaud JM, Light P (1992) Effects of K+ on the twitch and tetanic contraction in the sartorius muscle of the frog, Rana pipiens. Implications for fatigue in vivo. Can J Physiol Pharmacol 70:1236–1246
Sejersted OM, Hallen J (1987) Na+-K+ homeostasis of skeletal muscle during activation. Med Sport Sci 26:1–11
Sjøgaard G (1986) Water and electrolyte fluxes during exercise and their relation to muscle fatigue. Acta Physiol Scand 128: 129–136
Sjøgaard G (1988) Muscle energy metabolism and electrolyte shifts during low-level, prolonged, static contraction in man. Acta Physiol Scand 134:181 187
Sjogaard G (1990) Exercise-induced muscle fatigue-the significance of potassium. Acta Physiol Scand 140:5–63
Sjogaard G, Saltin B, Adams RP (1985) Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol 248:R190-R196
Stephens JA, Taylor A (1972) Fatigue of maintained voluntary muscle contraction in man. J Physiol (Lond) 220:1–18
Thomas CK, Woods JJ, Bigland-Ritchie B (1989) Impulse propagation and muscle activation in long maximal voluntary contractions. J Appl Physiol 67:1835–1842
Venosa RA, Horowicz P (1981) Density and apparent location of the sodium-pump in frog sartorius muscle. J Membr Biol 59:225–232
Vyskocil F, Hnik P, Rehfeldt H, Ujec E, Vejsada R (1983) The measurement of K+ concentration changes in human muscles during volitional contractions. Pflügers Arch 399:235–237
West W, Hicks A, Clements L, Dowling J (1995) The relationship between voluntary EMG, endurance time, and intensity of effort in isometric handgrip exercise. Eur J Appl Physiol 71:301–305
Westerblad H, Lee JA, Lamb AG, Bolsover SR, Allen DG (1990) Spatial gradients of intracellular calcium in skeletal muscle during fatigue. Pflügers Arch 415:734–740
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
West, W., Hicks, A., McKelvie, R. et al. The relationship between plasma potassium, muscle membrane excitability and force following quadriceps fatigue. Pflügers Arch — Eur J Physiol 432, 43–49 (1996). https://doi.org/10.1007/s004240050103
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s004240050103