Abstract
Main conclusion
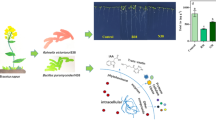
A natural rice rhizospheric isolate abates arsenic uptake in rice by increasing Fe plaque formation on rice roots.
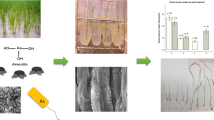
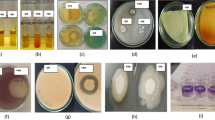
Rice (Oryza sativa L.) is the staple food for over half of the world’s population, but its quality and yield are impacted by arsenic (As) in some regions of the world. Bacterial inoculants may be able to mitigate the negative impacts of arsenic assimilation in rice, and we identified a nonpathogenic, naturally occurring rice rhizospheric bacterium that decreases As accumulation in rice shoots in laboratory experiments. We isolated several proteobacterial strains from a rice rhizosphere that promote rice growth and enhance the oxidizing environment surrounding rice root. One Pantoea sp. strain (EA106) also demonstrated increased iron (Fe)-siderophore in culture. We evaluated EA106’s ability to impact rice growth in the presence of arsenic, by inoculation of plants with EA106 (or control), subsequently grew the plants in As-supplemented medium, and quantified the resulting plant biomass, Fe and As concentrations, localization of Fe and As, and Fe plaque formation in EA106-treated and control plants. These results show that both arsenic and iron concentrations in rice can be altered by inoculation with the soil microbe EA106. The enhanced accumulation of Fe in the roots and in root plaques suggests that EA106 inoculation improves Fe uptake by the root and promotes the formation of a more oxidative environment in the rhizosphere, thereby allowing more expansive plaque formation. Therefore, this microbe may have the potential to increase food quality through a reduction in accumulation of toxic As species within the aerial portions of the plant.






Similar content being viewed by others
References
Abedin J, Cresser M, Meharg AA, Feldmann J, Cotter-Howells J (2002) Arsenic accumulation and metabolism in rice (Oryza sativa L.). Environ Sci Technol 36:962–968
Armstrong J, Armstrong W (1988) Phragmites australis—A preliminary study of soil oxidizing sites and internal gas transport pathways. New Phytol 108:373–382
Armstrong J, Armstrong W (2001) Rice and phragmites: effects of organic acids on growth, root permeability, and radial oxygen loss to the rhizosphere. Am J Bot 88:1359–1370
Banerjee M, Banerjee N, Bhattacharjee P, Mondal D, Lythgoe PR, Martinez M, Pan J, Polya DA, Giri AK (2013) High arsenic in rice is associated with elevated genotoxic effects in humans. Sci Rep 3:2195
Bar-Ness E, Hadar Y, Romheld CV, Marschner H (1992) Short-term effects of rhizosphere microorganisms on Fe uptake from microbial siderophores by maize and oat. Plant Physiol 100:451–456
Berendsen RL, Pieterse CMJ, Bakker P (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Beringer JE (1974) R factor transfer in Rhizobium leguminosarum. J Gen Microbiol 84:188–198
Bertani G (1951) Studies on lysogenesis I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62:293–300
Bissen M, Frimmel FH (2003) Arsenic—a review part I: occurrence, toxicity, speciation, mobility. Acta Hydrochem Hydrobiol 31:9–18
Budzikkiewicz H (1993) Secondary metabolites from fluorescent Pseudomonas. FEMS Microbiol Rev 104:209–228
Carson KC, Meyer JM, Dilworth MJ (2000) Hydroxamate siderophores of root nodule bacteria. Soil Biol Biochem 32:11–21
Chakraborti D, Rahman MM, Mukherjee A, Alauddin M, Hassan M, Dutta RN, Pati S, Mukherjee SC, Roy S, Quamruzzman Q, Rahman M, Morshed S, Islam T, Sorif S, Selim M, Islam RM, Hossain MM (2015) Groundwater arsenic contamination in Bangladesh-21 years of research. J Trace Elements Med Biol. doi:10.1016/j.jtemb.2015.01.003
Chen CC, Dixon JB, Turner FT (1980) Iron coatings on rice roots: morphology and models of development. Soil Sci Soc Am J 44:1113–1119
Dastager SG, Deepa CK, Pandey A (2010) Potential plant growth promoting activity of Serratia nematophila NII- 0.928 on black pepper (Piper nigrum L.). World J Microbiol Biotechnol 27:259–265
Dittmar J, Voegelin A, Roberts LC, Hug SJ, Saha GC, Ali MA, Borhan A, Badruzzaman M, Kretzschmar R (2007) Spatial distribution and temporal variability of arsenic in irrigated rice fields in Bangladesh. Environ Sci Technol 41:5967–5972
Dotaniya ML, Prasad D, Meena HM, Jajoria DK, Narolia GP, Pingoliya KK, Meena OP, Kumar K, Meena BP, Ram A, Das H, Sreenivasa M, Chari MS, Pal S (2013) Influence of phytosiderophore on iron and zinc uptake and rhizospheric microbial activity. Afr J Microbiol Res 7:5781–5788
Feng Y, Shen D, Song W (2006) Rice endophyte P. agglomerans YS19 promotes host plant growth and affects allocations of host photosynthates. J Appl Microbiol 100:938–945
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica. doi:10.6064/2012/963401
Hider RC, Kong X (2010) Chemistry and biology of siderophores. Nat Pro Rep 27:637–657
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ 347:1–32
Hu Y, Li JH, Zhu YG, Huang YZ, Hu HQ, Christie P (2005) Sequestration of As by iron plaque on the roots of three rice (Oryza sativa L.) cultivars in a low-P soil with or without P fertilizer. Environ Geochem Health 27:169–176
Huang H, Zhu YG, Chen Z, Yin X, Sun G (2012) Arsenic mobilization and speciation during iron plaque decomposition in a paddy soil. J Soils Sediments 12:402–410
Inoue H, Kobayashi T, Nozoye T, Takahashi M, Kakei Y, Suzuki K, Nakazono M, Nakanishi H, Mori S, Nishizawa NK (2009) Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J Biol Chem 284:3470–3479
Iremonger SF, Kelly DL (1988) The responses of four Irish wetland tree species to raised soil water levels. New Phytol 109:491–497
Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Wada Y, Watanabe S, Matsuhashi S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Rice plants take up iron as an Fe3+ -phytosiderophore and as Fe2+. Plant J 45:335–346
Islam FS, Gault AG, Boothman C, Polya DA, Charnock JM, Chatterjee D, Lloyd JR (2004) Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430:68–71
Jia SH, Gururanib MA, Chuna SC (2014) Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol Res 169:83–98
Kirk GJD (2003) Rice root properties for internal aeration and efficient nutrient acquisition in submerged soil. New Phytol 159:185–194
Laksmanan V, Selvaraj G, Bais H (2014) Functional soil microbiome: belowground solutions to an aboveground problem. Plant Physiol 166:689–700
Lee S, An G (2009) Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ 32:408–416
Lee S, Chiecko JC, Kim SA, Walker EL, Lee Y, Guerinot ML, An G (2009) Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiol 150:786–800
Li W, Ye Z, Wong M (2009) Metal mobilization and production of short-chain organic acids by rhizosphere bacteria associated with a Cd/Zn hyperaccumulating plant, Sedum alfredii. Plant Soil 326:453–467
Loaces I, Ferando L, Femández Scavino A (2011) Dynamics, diversity and tunction of endophytic siderophore-producing bacteria in dce. Microb Ecol 61:606–618
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FF (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105:9931–9935
Ma JF, Yamaji N, Mitani-Ueno N (2011) Transport of silicon from roots to panicles in plants. Proc Jpn Acad B Phys Biol Sci 87:377–385
Manninen M, Sandholm TM (1994) Methods for the detection of Pseudomonas siderophores. J Microbiol Methods 19:223–234
Meharg AA (2004) Arsenic in rice–understanding a new disaster for South-East Asia. Trends Plant Sci 9:415–417
Nair A, Juwarkar AA, Singh SK (2007) Production and characterization of siderophores and its application in arsenic removal from contaminated soil. Water Air Soil Poll 180:199–212
Ninno C, Dorosh PA (2001) Averting a food crisis: private imports and public targeted distribution in Bangladesh after the 1988 flood. Agricu Econ 25:203–207
Nordstrom DK (2002) The Questa baseline and pre-mining ground-water quality investigation [abs.]: Geol Soc Am Abstr Progr, vol 34, no. 6, p 51
Panaullah GM, Alam T, Hossain MB, Loeppert RH, Lauren GJ, Lauren Meisner CA, Ahmed ZU, Duxbury JM (2009) Arsenic toxicity to rice (Oryza sativa) in Bangladesh. Plant Soil 31:31–39
Payne SM (1994) Detection, isolation and characterization of siderophores. Methods Enzymol 235:329–344
Rahman MM, Mondal D, Das B, Sengupta MK, Ahamed S, Hossain MA, Samal AC, Sahae KC, Mukherjeef SC, Duttag RN, Chakraborti D (2014) Status of groundwater arsenic contamination in all 17 blocks of Nadia district in the state of West Bengal, India: A 23-year study report. J Hydrol 518:363–372
Rajkumar M, Ae N, Prasad MN, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trend Biotechnol 28:142–149
Reddy KR, De Laune RD (2008) Biogeochemistry of wetlands: science and applications. CRC Press, Boca Raton
Roberts LC, Hug SJ, Dittmar J, Voegelin A, Saha GC, Ali A, Borhan A, Badruzzaman M, Kretzschmar R (2007) Spatial distribution and temporal variability of arsenic in irrigated rice fields in Bangladesh 1. Irrigation water. Environ Sci Technol 41:5960–5966
Romheld V, Marschner H (1986) Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol 80:175–180
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Seyfferth AL, Fendorf S (2012) Silicate mineral impacts on the uptake and storage of arsenic and plant nutrients in rice (Oryza sativa L.). Environ Sci Technol 46:13176–13183
Seyfferth AL, Webb SM, Andrews JC, Fendorf S (2010) Arsenic localization, speciation, and co-occurrence with iron on rice (Oryza sativa L.) roots having variable Fe coatings. Environ Sci Technol 44:8108–8113
Seyfferth AL, Webb SM, Andrews JC, Fendorf S (2011) Defining the distribution of arsenic species and plant nutrients in rice (Oryza sativa L.) from the root to the grain. Geochim Cosmochim Ac 75:6655–6671
Song WY, Yamaki T, Yamaji N, Ko D, Jung KH, Fujii-Kashino M, An G, Martinoia E, Lee Y, Ma JF (2014) A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc Natl Acad Sci USA 111:15699–15704
Spence C, Alff E, Johnson C, Ramos C, Donofrio N, Sundaresan V, Bais HP (2014) Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol 14:130–147
Takahashi Y, Minamikawa R, Hattori KH, Kurishima K, Kihou N, Yuitta K (2004) Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ Sci Technol 38:1038–1044
Tanaka A, Loe R, Navasero SA (1966) Some mechanisms involved in the development in iron toxicity symptoms in the rice plant. Soil Sci Plant Nutr 12:158–164
Taylor GJ, Crowder AA (1983) Use of DCB technique for extraction of hydrous iron oxides from roots of wetland plants. Am J Bot 70:1254–1257
van Loon LC (2007) Plant responses to plant growth-promoting bacteria. Eur J Plant Pathol 119:243–254
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
von Wirén N, Klair S, Bansal S, Briat JF, Khodr H, Shioiri T, Leigh RA, Hider RC (1999) Nicotianamine chelates both FeIII and FeII. Implications for metal transport in plants. Plant Physiol 119:1107–1114
Wahlund TM, Madigan MT (1995) Genetic transfer by conjugation in the thermophilic photosynthetic green bacterium, Chlorobium tepidum. J Bacteriol 177:2583–2588
Webb SM (2006) SMAK: Sam’s Microprobe Analysis kit, vol 46, Stanford Synchrotron Radiation Laboratory
Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA (2007) Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol 41:6854–6859
Winkelmann G (1991) Specificity of iron transport in bacteria and fungi. In: Winkelmann G (ed) Handbook of microbial iron chelates. CRC Press Inc, Boca Raton, pp 65–105
Zhao FJ, Ago Y, Mitani N, Li RY, Su YH, Yamaji N, McGrath SP, Ma JF (2010a) The role of the rice aquaporin Lsi1 in arsenite efflux from roots. New Phytol 186:392–399
Zhao FJ, McGrath SP, Meharg AA (2010b) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559
Zheng R, Sun G, Zhu Y (2013) Effects of microbial processes on the fate of arsenic in paddy soil. Chin Sci Bull 58:186–193
Acknowledgments
We thank Samuel M. Webb (beam line 10–2 and 2–3 for assistance with XRF imaging and µXRD analyses. The authors also thank Dr. Jeffrey L. Caplan and the faculty Deborah Powell and Rebekah R. Helton, Bio-imaging center, Delaware Biotechnology Institute for their help with microscopic studies. H. P. B. acknowledges the support from NSF Award 0923806. H. P. B and D. L. S. acknowledge the support from DE-EPSCoR program, and A. L. S. acknowledges support from NSF Award 1338389. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515.
Ethical standard
We have followed all the guidelines of the Committee on Publication Ethics (COPE).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lakshmanan, V., Shantharaj, D., Li, G. et al. A natural rice rhizospheric bacterium abates arsenic accumulation in rice (Oryza sativa L.). Planta 242, 1037–1050 (2015). https://doi.org/10.1007/s00425-015-2340-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2340-2