Abstract
Main conclusion
Present review provides a thorough insight on some significant aspects of CHSs over a period of about past three decades with a better outlook for future studies toward comprehending the structural and mechanistic intricacy of this symbolic enzyme.
Abstract
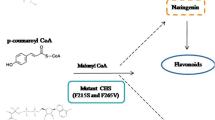
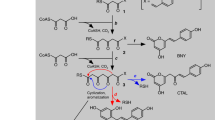
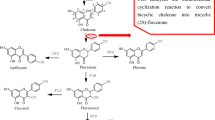
Polyketide synthases (PKSs) form a large family of iteratively acting multifunctional proteins that are involved in the biosynthesis of spectrum of natural products. They exhibit remarkable versatility in the structural configuration and functional organization with an incredible ability to generate different classes of compounds other than the characteristic secondary metabolite constituents. Architecturally, chalcone synthase (CHS) is considered to be the simplest representative of Type III PKSs. The enzyme is pivotal for phenylpropanoid biosynthesis and is also well known for catalyzing the initial step of the flavonoid/isoflavonoid pathway. Being the first Type III enzyme to be discovered, CHS has been subjected to ample investigations which, to a greater extent, have tried to understand its structural complexity and promiscuous functional behavior. In this context, we vehemently tried to collect the fragmented information entirely focussed on this symbolic enzyme from about past three–four decades. The aim of this review is to selectively summarize data on some of the fundamental aspects of CHSs viz, its history and distribution, localization, structure and analogs in non-plant hosts, promoter analyses, and role in defense, with an emphasis on mechanistic studies in different species and vis-à-vis mutation-led changes, and evolutionary significance which has been discussed in detail. The present review gives an insight with a better perspective for the scientific community for future studies devoted towards delimiting the mechanistic and structural basis of polyketide biosynthetic machinery vis-à-vis CHS.






Similar content being viewed by others
References
Abe I, Morita H (2010a) Structure and function of the chalcone synthase superfamily of plant type III polyketide synthases. Nat Prod Rep 27:809–838
Abe I, Morita HJNpr (2010b) Structure and function of the chalcone synthase superfamily of plant type III polyketide synthases. Nat Prod Rep 27:809–838
Abe I, Morita H, Nomura A, Noguchi H (2000) Substrate specificity of chalcone synthase: enzymatic formation of unnatural polyketides from synthetic cinnamoyl-CoA analogues. J Am Chem Soc 122:11242–11243
Abe I, Sano Y, Takahashi Y, Noguchi H (2003a) Site-directed mutagenesis of benzalacetone synthase the role of PHE215 in plant type III polyketide synthases. J Biol Chem 278:25218–25226
Abe I, Takahashi Y, Lou W, Noguchi HJOL (2003b) Enzymatic formation of unnatural novel polyketides from alternate starter and nonphysiological extension substrate by chalcone synthase. Org Lett 5:1277–1280
Abe T, Noma H, Noguchi H, Abe IJTl (2006) Enzymatic formation of an unnatural methylated triketide by plant type III polyketide synthases. Tetrahedron Lett 47:8727–8730
Adami C (2006) Reducible complexity. Science 312:61–63
Aharoni A, Gaidukov L, Khersonsky O, Gould SM, Roodveldt C, Tawfik DS (2005) The’evolvability’of promiscuous protein functions. Nat Genet 37:73–76
Ali MB, Hahn E-J, Paek K-Y (2007) Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules 12:607–621
Austin MB, Noel JP (2003) The chalcone synthase superfamily of type III polyketide synthases. Nat Prod Rep 20:79–110
Austin MB, Bowman ME, Ferrer J-L, Schröder J, Noel JP (2004) An aldol switch discovered in stilbene synthases mediates cyclization specificity of type III polyketide synthases. Chem Biol 11:1179–1194
Awasthi P, Mahajan V, Jamwal VL, Kapoor N, Rasool S, Bedi YS, Gandhi SG (2016) Cloning and expression analysis of chalcone synthase gene from Coleus forskohlii. J Genet 95:647–657
Beerhues L, Liu B (2009) Biosynthesis of biphenyls and benzophenones–Evolution of benzoic acid-specific type III polyketide synthases in plants. Phytochemistry 70:1719–1727
Bell J, Ryder T, Wingate V, Bailey J, Lamb C (1986) Differential accumulation of plant defense gene transcripts in a compatible and an incompatible plant-pathogen interaction. Mol Cell Biol 6:1615–1623
Bhan N, Cress BF, Linhardt RJ, Koffas M (2015) Expanding the chemical space of polyketides through structure-guided mutagenesis of Vitis vinifera stilbene synthase. Biochimie 115:136–143
Bhat WW, Dhar N, Razdan S, Rana S, Mehra R, Nargotra A, Dhar RS, Ashraf N, Vishwakarma R, Lattoo SK (2013) Molecular characterization of UGT94F2 and UGT86C4, two glycosyltransferases from Picrorhiza kurrooa: comparative structural insight and evaluation of substrate recognition. PLoS One 8:e73804
Bhat WW, Rana S, Dhar N, Razdan S, Pandith SA, Vishwakarma R, Lattoo SK (2014) An inducible NADPH–cytochrome P450 reductase from Picrorhiza kurrooa—an imperative redox partner of cytochrome P450 enzymes. Funct Integr Genomics 14:381–399
Bulgakov V, Tchernoded G, Mischenko N, Khodakovskaya M, Glazunov V, Radchenko S, Zvereva E, Fedoreyev S, Zhuravlev YN (2002) Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J Biotechnol 97:213–221
Burbulis IE, Iacobucci M, Shirley BW (1996) A null mutation in the first enzyme of flavonoid biosynthesis does not affect male fertility in Arabidopsis. Plant Cell 8:1013–1025
Cane DE, Walsh CT, Khosla C (1998) Harnessing the biosynthetic code: combinations, permutations, and mutations. Science 282:63–68
Chen S, Pan X, Li Y, Cui L, Zhang Y, Zhang Z, Pan G, Yang J, Cao P, Yang A (2017) Identification and characterization of chalcone synthase gene family members in Nicotiana tabacum. J Plant Growth Regul 36:374–384
Christensen AB, Gregersen PL, Schröder J, Collinge DB (1998) A chalcone synthase with an unusual substrate preference is expressed in barley leaves in response to UV light and pathogen attack. Plant Mol Biol 37:849–857
Creelman RA, Tierney ML, Mullet JE (1992) Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci 89:4938–4941
Dae-Yeon S, Fukuma K, Kagami J, Yamazaki Y, Shibuya M, Ebizuka Y, Sankawa U (2000) Identification of amino acid residues important in the cyclization reactions of chalcone and stilbene synthases. Biochem J 350:229–235
Dai M, Feng Y, Tonge PJ (2001) Synthesis of crotonyl-oxyCoA: a mechanistic probe of the reaction catalyzed by enoyl-CoA hydratase. J Am Chem Soc 123:506–507
Dao T, Linthorst H, Verpoorte R (2011) Chalcone synthase and its functions in plant resistance. Phytochem Rev 10:397–412
Dehghan S, Sadeghi M, Pöppel A, Fischer R, Lakes-Harlan R, Kavousi HR, Vilcinskas A, Rahnamaeian M (2014) Differential inductions of phenylalanine ammonia-lyase and chalcone synthase during wounding, salicylic acid treatment, and salinity stress in safflower, Carthamus tinctorius. Biosci Rep 34:e00114
Deng X, Bashandy H, Ainasoja M, Kontturi J, Pietiäinen M, Laitinen RA, Albert VA, Valkonen J, Elomaa P, Teeri TH (2014) Functional diversification of duplicated chalcone synthase genes in anthocyanin biosynthesis of Gerbera hybrida. New Phytol 201:1469–1483
Des Marais DL, Rausher MD (2008) Escape from adaptive conflict after duplication in an anthocyanin pathway gene. Nature 454:762–765
Dhar N, Rana S, Razdan S, Bhat WW, Hussain A, Dhar RS, Vaishnavi S, Hamid A, Vishwakarma R, Lattoo SK (2014) Cloning and functional characterization of three branch point oxidosqualene cyclases from Gerbera hybrida (L.) dunal. J Biol Chem 289:17249–17267
Dhawale S, Souciet G, Kuhn DN (1989) Increase of chalcone synthase mRNA in pathogen-inoculated soybeans with race-specific resistance is different in leaves and roots. Plant Physiol 91:911–916
Dibyendu DM (2015) A brief review on plant type III polyketide synthases, an important group of enzyme of secondary metabolism. Res J Recent Sci 4(10):138–147
Dixon RA (2001) Phytochemistry in the genomics and post-genomics eras. Pergamon Press, Oxford, New York, pp 1–258
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Dobritsa AA, Lei Z, Nishikawa S-i, Urbanczyk-Wochniak E, Huhman DV, Preuss D, Sumner LW (2010) LAP5 and LAP6 encode anther-specific proteins with similarity to chalcone synthase essential for pollen exine development in Arabidopsis. Plant Physiol 153:937–955
Durbin ML, Learn GH, Huttley GA, Clegg MT (1995) Evolution of the chalcone synthase gene family in the genus Ipomoea. Proc Natl Acad Sci 92:3338–3342
Erdmann R, Kunau WH (1994) Purification and immunolocalization of the peroxisomal 3-oxoacyl-coA thiolase from Saccharomyces cerevisiae. Yeast 10:1173–1182
Erdtman G (1952) Pollen morphology and plant taxonomy: an introduction to palynology. Hafner Publishing Company, New York, pp 1–553
Feinbaum RL, Storz G, Ausubel FM (1991) High intensity and blue light regulated expression of chimeric chalcone synthase genes in transgenic Arabidopsis thaliana plants. Mol Gen Genet MGG 226:449–456
Ferrer J-L, Jez JM, Bowman ME, Dixon RA, Noel JP (1999) Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat Struct Mol Biol 6:775–784
Fliegmann J, Schröder G, Schanz S, Britsch L, Schröder J (1992) Molecular analysis of chalcone and dihydropinosylvin synthase from Scots pine (Pinus sylvestris), and differential regulation of these and related enzyme activities in stressed plants. Plant Mol Biol 18:489–503
Flores-Sanchez IJ, Verpoorte R (2008) PKS activities and biosynthesis of cannabinoids and flavonoids in Cannabis sativa L. plants. Plant Cell Physiol 49:1767–1782
Flores-Sanchez IJ, Verpoorte R (2009) Plant polyketide synthases: a fascinating group of enzymes. Plant Physiol Biochem 47:167–174
Fritze K, Staiger D, Czaja I, Walden R, Schell J, Wing D (1991) Developmental and UV Light regulation of the snapdragon chalcone synthase promoter. Plant Cell 3:893–905
Fukuma K, Neuls ED, Ryberg JM, Suh D-Y, Sankawa U (2007) Mutational analysis of conserved outer sphere arginine residues of chalcone synthase. J Biochem 142:731–739
Funa N, Ohnishi Y, Fujii I, Shibuya M, Ebizuka Y, Horinouchi S (1999) A new pathway for polyketide synthesis in microorganisms. Nature 400:897–899
Girija A, Devakumar LJPS, Vijayanathan M, Vasudevan SE (2017) Nitric oxide as a bioactive molecule in the regulation of chalcone synthase during jasmonic acid mediated defense signaling in ginger. Plant Cell Tissue Organ Cult (PCTOC) 128:715–721
Glagoleva AY, Ivanisenko NV, Khlestkina EK (2019) Organization and evolution of the chalcone synthase gene family in bread wheat and relative species. BMC Genet 20:1–9
Gläßgen WE, Rose A, Madlung J, Koch W, Gleitz J, Seitz HU (1998) Regulation of enzymes involved in anthocyanin biosynthesis in carrot cell cultures in response to treatment with ultraviolet light and fungal elicitors. Planta 204:490–498
Gokhale RS, Sankaranarayanan R, Mohanty D (2007) Versatility of polyketide synthases in generating metabolic diversity. Curr Opin Struct Biol 17:736–743
Gross H (1912) Arbeit aus d. Botan. Inst. d. Kgl. Albertus-Univ. zu Königsberg iP: Beiträge zur Kenntnis der Polygonaceen. PhD dissertation. W. Engelmann
Gross F, Luniak N, Perlova O, Gaitatzis N, Jenke-Kodama H, Gerth K, Gottschalk D, Dittmann E, Müller R (2006) Bacterial type III polyketide synthases: phylogenetic analysis and potential for the production of novel secondary metabolites by heterologous expression in pseudomonads. Arch Microbiol 185:28–38
Gu Z, Men S, Zhu J, Hao Q, Tong N, Liu Z-A, Zhang H, Shu Q, Wang L (2019) Chalcone synthase is ubiquitinated and degraded via interactions with a RING-H2 protein in petals of Paeonia hybrid ‘He Xie’. J Exp Bot. https://doi.org/10.1093/jxb/erz245
Hahlbrock K, Scheel D (1989) Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Biol 40:347–369
Han Y-Y, Ming F, Wang W, Wang J-W, Ye M-M, Shen D-L (2006) Molecular evolution and functional specialization of chalcone synthase superfamily from Phalaenopsis orchid. Genetica 128:429–438
Han Y, Zhao W, Wang Z, Zhu J, Liu Q (2014) Molecular evolution and sequence divergence of plant chalcone synthase and chalcone synthase-Like genes. Genetica 142:215–225
Han Y, Ding T, Su B, Jiang H (2016) Genome-wide identification, characterization and expression analysis of the chalcone synthase family in maize. Int J Mol Sci 17:1–15
Han Y, Cao Y, Jiang H, Ding T (2017) Genome-wide dissection of the chalcone synthase gene family in Oryza sativa. Mol Breeding 37:1–12
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504
Harrison M (1993) Isoflavonoid accumulation and expression of defense gene transcripts during the establishment of vesicular arbuscular mycorrhizal associations in roots of medicago trancatula. Mol Plant Microbe Interact 6:643–654
Hartmann U, Valentine WJ, Christie JM, Hays J, Jenkins GI, Weisshaar B (1998) Identification of UV/blue light-response elements in the Arabidopsis thaliana chalcone synthase promoter using a homologous protoplast transient expression system. Plant Mol Biol 36:741–754
Hashimoto M, Koen T, Takahashi H, Suda C, Kitamoto K, Fujii I (2014a) Aspergillus oryzae CsyB catalyzes the condensation of two β-ketoacyl-CoAs to form 3-acetyl-4-hydroxy-6-alkyl-α-pyrone. J Biol Chem 289:19976–19984
Hashimoto M, Nonaka T, Fujii I (2014b) Fungal type III polyketide synthases. Nat Prod Rep 31:1306–1317
Haussühl K, Rohde W, Weissenboeck G (1996) Expression of chalcone synthase genes in coleoptiles and primary leaves of Secale cereale L. after induction by UV radiation: evidence for a UV-protective role of the coleoptile. Botanica Acta 109:229–238
Hectors K, van Oevelen S, Guisez Y, Prinsen E, Jansen MA (2012) The phytohormone auxin is a component of the regulatory system that controls UV-mediated accumulation of flavonoids and UV-induced morphogenesis. Physiol Plant 145:594–603
Helariutta Y, Kotilainen M, Elomaa P, Kalkkinen N, Bremer K, Teeri TH, Albert VA (1996) Duplication and functional divergence in the chalcone synthase gene family of Asteraceae: evolution with substrate change and catalytic simplification. Proc Natl Acad Sci 93:9033–9038
Heller W, Hahlbrock K (1980) Highly purified “flavanone synthase” from parsley catalyzes the formation of naringenin chalcone. Arch Biochem Biophys 200:617–619
Hinderer W, Seitz HU (1985) Chalcone synthase from cell suspension cultures of Daucus carota L. Arch Biochem Biophys 240:265–272
Holding DR, Meeley RB, Hazebroek J, Selinger D, Gruis F, Jung R, Larkins BA (2010) Identification and characterization of the maize arogenate dehydrogenase gene family. J Exp Bot 61(13):3663–3673
Hrazdina G, Kreuzaler F, Hahlbrock K, Grisebach H (1976) Substrate specificity of flavanone synthase from cell suspension cultures of parsley and structure of release products in vitro. Arch Biochem Biophys 175:392–399
Hu L, He H, Zhu C, Peng X, Fu J, He X, Chen X, Ouyang L, Bian J, Liu S (2017) Genome-wide identification and phylogenetic analysis of the chalcone synthase gene family in rice. J Plant Res 130:95–105
Ito M, Ichinose Y, Kato H, Shiraishi T, Yamada T (1997) Molecular evolution and functional relevance of the chalcone synthase genes of pea. Mol Gen Genet MGG 255:28–37
Jähne A, Fritzen C, Weissenböck G (1993) Chalcone synthase and flavonoid products in primary-leaf tissues of rye and maize. Planta 189:39–46
Jez JM, Noel JP (2000) Mechanism of chalcone synthase pK a of the catalytic cysteine and the role of the conserved histidine in a plant polyketide synthase. J Biol Chem 275:39640–39646
Jez JM, Austin MB, Ferrer J-L, Bowman ME, Schröder J, Noel JP (2000a) Structural control of polyketide formation in plant-specific polyketide synthases. Chem Biol 7:919–930
Jez JM, Ferrer J-L, Bowman ME, Dixon RA, Noel JP (2000b) Dissection of malonyl-coenzyme A decarboxylation from polyketide formation in the reaction mechanism of a plant polyketide synthase. Biochemistry 39:890–902
Jez JM, Bowman ME, Noel JP (2002) Expanding the biosynthetic repertoire of plant type III polyketide synthases by altering starter molecule specificity. Proc Natl Acad Sci 99:5319–5324
Jiang C, Schommer CK, Kim SY, Suh D-Y (2006) Cloning and characterization of chalcone synthase from the moss, Physcomitrella patens. Phytochemistry 67:2531–2540
Jiang C, Kim SY, Suh D-Y (2008) Divergent evolution of the thiolase superfamily and chalcone synthase family. Mol Phylogenet Evol 49:691–701
Junghans H, Dalkin K, Dixon RA (1993) Stress responses in alfalfa (Medicago sativa L.). 15. Characterization and expression patterns of members of a subset of the chalcone synthase multigene family. Plant Mol Biol 22:239–253
Juvvadi PR, Seshime Y, Kitamoto K (2005) Genomics reveals traces of fungal phenylpropanoidflavonoid metabolic pathway in the filamentous fungus Aspergillus oryzae. J Microbiol (Seoul, Korea) 43:475–486
Kays SJ, Paull RE, Mohammed M, Benítez C, Castro H, Ricca A, Vaudagna S, Bal L, Oi D, Mau R (2004) Postharvest biology. IICA, Port of Spain, Trinidad and Tobago
Kehrel B, Wiermann R (1985) Immunochemical localization of phenylalanine ammonia-lyase and chalcone synthase in anthers. Planta 163:183–190
Khalil AS, Collins JJ (2010) Synthetic biology: applications come of age. Nat Rev Genet 11:367–379
Koch MA, Weisshaar B, Kroymann J, Haubold B, Mitchell-Olds T (2001) Comparative genomics and regulatory evolution: conservation and function of the Chs and Apetala3 promoters. Mol Biol Evol 18:1882–1891
Koes RE, Spelt CE, Mol JN, Gerats AG (1987) The chalcone synthase multigene family of Petunia hybrida (V30): sequence homology, chromosomal localization and evolutionary aspects. Plant Mol Biol 10:159–169
Koes RE, Spelt CE, Mol JN (1989a) The chalcone synthase multigene family of Petunia hybrida (V30): differential, light-regulated expression during flower development and UV light induction. Plant Mol Biol 12:213–225
Koes RE, Spelt CE, van den Elzen PJ, Mol JN (1989b) Cloning and molecular characterization of the chalcone synthase multigene family of Petunia hybrida. Gene 81:245–257
Kosová K, Vítámvás P, Urban M, Klíma M, Roy A, Prášil I (2015) Biological networks underlying abiotic stress tolerance in temperate crops—a proteomic perspective. Int J Mol Sci 16:20913–20942
Kotopka BJ, Li Y, Smolke CD (2018) Synthetic biology strategies toward heterologous phytochemical production. Nat Prod Rep 35:902–920
Kreuzaler F, Hahlbrock K (1972) Enzymatic synthesis of aromatic compounds in higher plants: formation of naringenin (5, 7, 4′-trihydroxyflavanone) from p-coumaroyl coenzyme A and malonyl coenzyme A. FEBS Lett 28:69–72
Kreuzaler F, Hahlbrock K (1975) Enzymic synthesis of an aromatic ring from acetate units. Eur J Biochem 56:205–213
Kreuzaler F, Light RJ, Hahlbrock K (1978) Flavanone synthase catalyzes CO2 exchange and decarboxylation of malonyl-CoA. FEBS Lett 94:175–178
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Lamb CJ, Lawton MA, Dron M, Dixon RA (1989) Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell 56:215–224
Lelli KM, Slattery M, Mann RS (2012) Disentangling the many layers of eukaryotic transcriptional regulation. Annu Rev Genet 46:43–68
Li AJ (1998) Polygonaceae Juss. In: Li AJ (ed) Flora Reipublicae Popularis Sinicae, vol 25. 1. Science Press, Beijing, pp 1–237 (ISBN 703006450X)
Li J, Zhang J (1983) Investigation on origin and quality of commodities of rhubarb. Chin J Pharm Anal 3:333–339
Lijuan C, Huiming G, Yi L, Hongmei C (2015) Chalcone synthase EaCHS1 from Eupatorium adenophorum functions in salt stress tolerance in tobacco. Plant Cell Rep 34:885–894
Lim Y, Go M, Yew W (2016) Exploiting the biosynthetic potential of type III polyketide synthases. Molecules 21:1–37
Liou G, Chiang Y-C, Wang Y, Weng J-K (2018) Mechanistic basis for the evolution of chalcone synthase catalytic cysteine reactivity in land plants. J Biol Chem 293:18601–18612
Liu B, Falkenstein-Paul H, Schmidt W, Beerhues L (2003) Benzophenone synthase and chalcone synthase from Hypericum androsaemum cell cultures: cDNA cloning, functional expression, and site-directed mutagenesis of two polyketide synthases. Plant J 34:847–855
Liu B, Raeth T, Beuerle T, Beerhues L (2007) Biphenyl synthase, a novel type III polyketide synthase. Planta 225:1495–1503
Liu X, Meng X, Ye J, Zhang W, Chang J, Xu F (2018) Characterization of novel chalcone synthase gene (CnCHS) from Chamaemelum nobile. Biotechnology 17:54–61
Lu Y, Rausher MD (2003) Evolutionary rate variation in anthocyanin pathway genes. Mol Biol Evol 20:1844–1853
Lu X, Zhou W, Gao F (2009) Cloning, characterization and localization of CHS gene from blood orange, Citrus sinensis (L.) Osbeck cv. Ruby. Mol Biol Rep 36:1983–1990
Ma L-Q, Pang X-B, Shen H-Y, Pu G-B, Wang H-H, Lei C-Y, Wang H, Li G-F, Liu B-Y, Ye H-C (2009) A novel type III polyketide synthase encoded by a three-intron gene from Polygonum cuspidatum. Planta 229:457–469
Martin C (1993) Structure, function, and regulation of the chalcone synthase. Int Rev Cytol 147:233–284
Mathieu M, Zeelen J, Pauptit R, Erdmann R, Kunau W, Wierenga R (1994) The 2.8 Å crystal structure of peroxisomal 3-ketoacyl-CoA thiolase of Saccharomyces cerevisiae: a five-layered αβαβα structure constructed from two core domains of identical topology. Structure 2:797–808
Mo Y, Nagel C, Taylor LP (1992) Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proc Natl Acad Sci 89:7213–7217
Moche M, Dehesh K, Edwards P, Lindqvist Y (2001) The crystal structure of β-ketoacyl-acyl carrier protein synthase II from Synechocystis sp. at 1.54 Å resolution and its relationship to other condensing enzymes. J Mol Biol 305:491–503
Mondal M (1997) Pollen morphology and systematic relationship of the family Polygonaceae. Botanical Survey of India, India
Moore BS, Hopke JN (2001) Discovery of a new bacterial polyketide biosynthetic pathway. ChemBioChem 2:35–38
Moore BS, Hertweck C, Hopke JN, Izumikawa M, Kalaitzis JA, Nilsen G, O’Hare T, Piel J, Shipley PR, Xiang L (2002) Plant-like biosynthetic pathways in bacteria: from benzoic acid to chalcone 1. J Nat Prod 65:1956–1962
Morita H, Takahashi Y, Noguchi H, Abe I (2000) Enzymatic formation of unnatural aromatic polyketides by chalcone synthase. Biochem Biophys Res Commun 279:190–195
Nagy NE, Fossdal CG, Krokene P, Krekling T, Lönneborg A, Solheim H (2004) Induced responses to pathogen infection in Norway spruce phloem: changes in polyphenolic parenchyma cells, chalcone synthase transcript levels and peroxidase activity. Tree Physiol 24:505–515
Nair DR, Anand S, Verma P, Mohanty D, Gokhale RS (2012) Genetic, biosynthetic and functional versatility of polyketide synthases. Curr Sci India 102:277–287
Novák P, Krofta K, Matousek J (2006) Chalcone synthase homologues from Humulus lupulus: some enzymatic properties and expression. Biol Plant 50:48–54
Oguro S, Akashi T, Ayabe S-i, Noguchi H, Abe IJB (2004) Probing biosynthesis of plant polyketides with synthetic N-acetylcysteamine thioesters. Biochem Biophys Res Commun 325:561–567
Pandith SA, Dhar N, Rana S, Bhat WW, Kushwaha M, Gupta AP, Shah MA, Vishwakarma R, Lattoo SK (2016) Functional promiscuity of two divergent paralogs of type III plant polyketide synthases. Plant Physiol 171:2599–2619
Pang Y, Shen G, Wu W, Liu X, Lin J, Tan F, Sun X, Tang K (2005) Characterization and expression of chalcone synthase gene from Ginkgo biloba. Plant Sci 168:1525–1531
Parvez A, Giri S, Giri GR, Kumari M, Bisht R, Saxena P (2018) Novel type III polyketide synthases biosynthesize methylated polyketides in Mycobacterium marinum. Sci Rep 8:1–13
Peters A, Schneider-Poetsch HA, Schwarz H, Weissenböck G (1988) Biochemical and immunological characterization of chalcone synthase from rye leaves. J Plant Physiol 133:178–182
Purnick PE, Weiss R (2009) The second wave of synthetic biology: from modules to systems. Nat Rev Mol Cell Biol 10:410–422
Radhakrishnan EK, Varghese RT, Vasudevan SE (2010) Unusual intron in the second exon of a type III polyketide synthase gene of Alpinia calcarata Rosc. Genet Mol Biol 33:141–145
Rana S, Bhat WW, Dhar N, Pandith SA, Razdan S, Vishwakarma R, Lattoo SK (2014) Molecular characterization of two A-type P450 s, WsCYP98A and WsCYP76A from Withania somnifera (L.) Dunal: expression analysis and withanolide accumulation in response to exogenous elicitations. BMC Biotechnol 14:1–17
Reimold U, Kröger M, Kreuzaler F, Hahlbrock K (1983) Coding and 3′ non-coding nucleotide sequence of chalcone synthase mRNA and assignment of amino acid sequence of the enzyme. EMBO J 2:1801–1805
Richard S, Lapointe G, Rutledge RG, Séguin A (2000) Induction of chalcone synthase expression in white spruce by wounding and jasmonate. Plant Cell Physiol 41:982–987
Rommeswinkel M, Karwatzki B, Beerhues L, Wiermann R (1992) Immunofluorescence localization of chalcone synthase in roots of Pisum sativum L. and Phaseolus vulgaris L. and comparable immunochemical analysis of chalcone synthase from pea leaves. Protoplasma 166:115–121
Ryder TB, Cramer CL, Bell JN, Robbins MP, Dixon RA, Lamb CJ (1984) Elicitor rapidly induces chalcone synthase mRNA in Phaseolus vulgaris cells at the onset of the phytoalexin defense response. Proc Natl Acad Sci 81:5724–5728
Ryder TB, Hedrick SA, Bell JN, Liang X, Clouse SD, Lamb CJ (1987) Organization and differential activation of a gene family encoding the plant defense enzyme chalcone synthase in Phaseolus vulgaris. Mol Gen Genet MGG 210:219–233
Saslowsky D, Winkel-Shirley B (2001) Localization of flavonoid enzymes in Arabidopsis roots. Plant J 27:37–48
Saslowsky DE, Warek U, Winkel BS (2005) Nuclear localization of flavonoid enzymes in Arabidopsis. J Biol Chem 280:23735–23740
Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci 97:11655–11660
Schijlen EG, de Vos CR, Martens S, Jonker HH, Rosin FM, Molthoff JW, Tikunov YM, Angenent GC, van Tunen AJ, Bovy AG (2007) RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits. Plant Physiol 144:1520–1530
Schmelzer E, Jahnen W, Hahlbrock K (1988) In situ localization of light-induced chalcone synthase mRNA, chalcone synthase, and flavonoid end products in epidermal cells of parsley leaves. Proc Natl Acad Sci 85:2989–2993
Schnitzler JP, Jungblut TP, Heller W, Kofferlein M, Hutzler P, Heinzmann U, Schmelzer E, Ernst D, Langebartels C, Sandermann H (1996) Tissue localization of UV-B-screening pigments and of chalcone synthase mRNA in needles of Scots pine seedlings. New Phytol 132:247–258
Schroder G, Brown JW, Schroder J (1988) Molecular analysis of resveratrol synthase: cDNA, genomic clones and relationship with chalcone synthase. Eur J Biochem 172:161–169
Seshime Y, Juvvadi PR, Fujii I, Kitamoto K (2005) Discovery of a novel superfamily of type III polyketide synthases in Aspergillus oryzae. Biochem Biophys Res Commun 331:253–260
Shen B (2000) Biosynthesis of aromatic polyketides. Biosynthesis. Springer, Berlin, pp 1–51
Shen B (2003) Polyketide biosynthesis beyond the type I, II and III polyketide synthase paradigms. Curr Opin Chem Biol 7:285–295
Shen Y, Li X, Chai T, Wang H (2016) Outer-sphere residues influence the catalytic activity of a chalcone synthase from Polygonum cuspidatum. FEBS Open Bio 6:610–618
Sievers F, Higgins DG (2014) Clustal omega, accurate alignment of very large numbers of sequences. In: Russell D (ed) Multiple sequence alignment methods. Methods in molecular biology (methods and protocols), vol 1079. Humana Press, Totowa, NJ, pp 105–116
Sommer H, Saedler H (1986) Structure of the chalcone synthase gene of Antirrhinum majus. Mol Gen Genet MGG 202:429–434
Springob K, Lukačin R, Ernwein C, Gröning I, Matern U (2000) Specificities of functionally expressed chalcone and acridone synthases from Ruta graveolens. FEBS J 267:6552–6559
Staiger D, Kaulen H, Schell J (1989) A CACGTG motif of the Antirrhinum majus chalcone synthase promoter is recognized by an evolutionarily conserved nuclear protein. Proc Natl Acad Sci 86:6930–6934
Staunton J, Weissman KJ (2001) Polyketide biosynthesis: a millennium review. Nat Prod Rep 18:380–416
Suh D-Y, Kagami J, Fukuma K, Sankawa U (2000) Evidence for catalytic cysteine–histidine dyad in chalcone synthase. Biochem Biophys Res Commun 275:725–730
Sun W, Meng X, Liang L, Jiang W, Huang Y, He J, Hu H, Almqvist J, Gao X, Wang L (2015) Molecular and biochemical analysis of chalcone synthase from Freesia hybrid in flavonoid biosynthetic pathway. PLoS One 10:e0119054
Taura F, Iijima M, Yamanaka E, Takahashi H, Kenmoku H, Saeki H, Morimoto S, Asakawa Y, Kurosaki F, Morita H (2016) A novel class of plant type III polyketide synthase involved in orsellinic acid biosynthesis from Rhododendron dauricum. Front Plant Sci 7:1–15
Tawfik DS, Khersonsky O (2010) Enzyme promiscuity: a mechanistic and evolutionary perspective. Annu Rev Biochem 79:471–505
Taylor L, Jorgensen R (1992) Conditional male fertility in chalcone synthase-deficient petunia. J Hered 83:11–17
Thain SC, Murtas G, Lynn JR, McGrath RB, Millar AJ (2002) The circadian clock that controls gene expression in Arabidopsis is tissue specific. Plant Physiol 130:102–110
Tian L, Wan S-B, Pan Q-H, Zheng Y-J, Huang W-D (2008) A novel plastid localization of chalcone synthase in developing grape berry. Plant Sci 175:431–436
Tian J, Shen H, Zhang J, Song T, Yao Y (2011) Characteristics of chalcone synthase promoters from different leaf-color Malus crabapple cultivars. Sci Hortic 129:449–458
Trezzini GF, Horrichs A, Somssich IE (1993) Isolation of putative defense-related genes from Arabidopsis thaliana and expression in fungal elicitor-treated cells. Plant Mol Biol 21:385–389
Tropf S, Lanz T, Rensing S, Schröder J, Schröder G (1994) Evidence that stilbene synthases have developed from chalcone synthases several times in the course of evolution. J Mol Evol 38:610–618
Tropf S, Kärcher B, Schröder G, Schröder J (1995) Reaction mechanisms of homodimeric plant polyketide synthases (stilbene and chalcone synthase). A single active site for the condensing reaction is sufficient for synthesis of stilbenes, chalcones, and 6′-deoxychalcones. J Biol Chem 270:7922–7928
Trujillo M (2017) News from the PUB: plant U-box type E3 ubiquitin ligases. J Exp Bot 69:371–384
Ueda K, Kim KM, Beppu T, Horinouchi S (1995) Overexpression of a gene cluster encoding a chalcone synthase-like protein confers redbrown pigment production in Streptomyces griseus. J Antibiot 48:638–646
Vadivel AKA, Krysiak K, Tian G, Dhaubhadel S (2018) Genome-wide identification and localization of chalcone synthase family in soybean (Glycine max [L] Merr). BMC Plant Biol 18:1–13
van der Meer IM, Spelt CE, Mol JN, Stuitje AR (1990) Promoter analysis of the chalcone synthase (chsA) gene of Petunia hybrida: a 67 bp promoter region directs flower-specific expression. Plant Mol Biol 15:95–109
VanEtten H, Matthews D, Matthews PS (1989) Phytoalexin detoxification: importance for pathogenicity and practical implications. Annu Rev Phytopathol 27:143–164
Wade HK, Bibikova TN, Valentine WJ, Jenkins GI (2001) Interactions within a network of phytochrome, cryptochrome and UV-B phototransduction pathways regulate chalcone synthase gene expression in Arabidopsis leaf tissue. Plant J 25:675–685
Wakil SJ (1989) Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry 28:4523–4530
Walhout AJ (2006) Unraveling transcription regulatory networks by protein–DNA and protein–protein interaction mapping. Genome Res 16:1445–1454
Walker JW (1974) Evolution of exine structure in the pollen of primitive angiosperms. Am J Bot 61:891–902
Wang H, Wang W, Zhan J, Yan A, Sun L, Zhang G, Wang X, Ren J, Huang W, Xu H (2016) The accumulation and localization of chalcone synthase in grapevine (Vitis vinifera L.). Plant Physiol Biochem 106:165–176
Wani TA, Pandith SA, Gupta AP, Chandra S, Sharma N, Lattoo SK (2017) Molecular and functional characterization of two isoforms of chalcone synthase and their expression analysis in relation to flavonoid constituents in Grewia asiatica L. PLoS One 12:e0179155
Wanibuchi K, Zhang P, Abe T, Morita H, Kohno T, Chen G, Noguchi H, Abe I (2007) An acridone-producing novel multifunctional type III polyketide synthase from Huperzia serrata. FEBS J 274:1073–1082
Weisshaar B, Jenkins GI (1998) Phenylpropanoid biosynthesis and its regulation. Curr Opin Plant Biol 1:251–257
Wingender R, Röhrig H, Höricke C, Wing D, Schell J (1989) Differential regulation of soybean chalcone synthase genes in plant defence, symbiosis and upon environmental stimuli. Mol Gen Genet MGG 218:315–322
Wolfrom ML (1959) Carbohydrate chemistry of substances of biological interest: symposium I: proceedings of the fourth international congress of biochemistry. Pergamon Press, Vienna, pp 1–6
Wu S, O’Leary SJ, Gleddie S, Eudes F, Laroche A, Robert LS (2008) A chalcone synthase-like gene is highly expressed in the tapetum of both wheat (Triticum aestivum L.) and triticale (× Triticosecale Wittmack). Plant Cell Rep 27:1441–1449
Yahyaa M, Ali S, Davidovich-Rikanati R, Ibdah M, Shachtier A, Eyal Y, Lewinsohn E, Ibdah M (2017) Characterization of three chalcone synthase-like genes from apple (Malus x domestica Borkh.). Phytochemistry 140:125–133
Yamazaki Y, Suh D-Y, Sitthithaworn W, Ishiguro K, Kobayashi Y, Shibuya M, Ebizuka Y, Sankawa U (2001) Diverse chalcone synthase superfamily enzymes from the most primitive vascular plant, Psilotum nudum. Planta 214:75–84
Yang WC, Cramers HCC, Hogendijk P, Katinakis P, Wijffelman CA, Franssen H, Van Kammen A, Bisseling T (1992) In-situ localization of chalcone synthase mRNA in pea root nodule development. Plant J 2:143–151
Yang M, Zhang D, Zheng J, Liu J (2001) Pollen morphology and its systematic and ecological significance in Rheum (Polygonaceae) from China. Nordic J Bot 21:411–418
Yilmaz Y, Toledo RT (2004) Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid. J Agric food Chem 52:255–260
Yokoigawa J, Morimoto K, Shiono Y, Uesugi S, Kimura K-i, Kataoka T (2015) Allantopyrone A, an α-pyrone metabolite from an endophytic fungus, inhibits the tumor necrosis factor α-induced nuclear factor κB signaling pathway. J Antibiot 68:71–75
Yu O, Jez JM (2008) Nature’s assembly line: biosynthesis of simple phenylpropanoids and polyketides. Plant J 54:750–762
Yu J, Bhatnagar D, Cleveland TE (2004) Completed sequence of aflatoxin pathway gene cluster in Aspergillus parasiticus1. FEBS Lett 564:126–130
Zhang X, Gou M, Liu C-J (2013) Arabidopsis Kelch repeat F-box proteins regulate phenylpropanoid biosynthesis via controlling the turnover of phenylalanine ammonia-lyase. Plant Cell 25:4994–5010
Zhang X, Gou M, Guo C, Yang H, Liu C-J (2015) Down-regulation of Kelch domain-containing F-box protein in Arabidopsis enhances the production of (poly) phenols and tolerance to ultraviolet radiation. Plant Physiol 167:337–350
Zhang X, Abrahan C, Colquhoun TA, Liu C-J (2017) A proteolytic regulator controlling chalcone synthase stability and flavonoid biosynthesis in Arabidopsis. Plant Cell 29:1157–1174
Zhang C, Yao X, Ren H, Wang K, Chang J (2019) Isolation and Characterization of three chalcone synthase genes in pecan (Carya illinoinensis). Biomolecules 9:1–12
Zhao N, Wang G, Norris A, Chen X, Chen F (2013) Studying plant secondary metabolism in the age of genomics. Crit Rev Plant Sci 32:369–382
Zheng Q, Huang T, Zhang L, Zhou Y, Luo H, Xu H, Wang X (2016) Dysregulation of ubiquitin-proteasome system in neurodegenerative diseases. Front Aging Neurosci 8:303
Zhou B, Li Y, Xu Z, Yan H, Homma S, Kawabata S (2007) Ultraviolet A-specific induction of anthocyanin biosynthesis in the swollen hypocotyls of turnip (Brassica rapa). J Exp Bot 58:1771–1781
Zhou X-M, Zheng C-J, Song X-P, Han C-R, Chen W-H, Chen G-Y (2014) Antibacterial α-pyrone derivatives from a mangrove-derived fungus Stemphylium sp. 33231 from the South China Sea. J Antibiot 67:401–403
Zhu Q, Dröge-Laser W, Dixon RA, Lamb C (1996) Transcriptional activation of plant defense genes. Curr Opin Genet Dev 6:624–630
Zobel AM, Hrazdina G (1992) Chalcone synthase localization in shoot apices of Fagopyrum, Brassica and Pisum. Ann Bot 70:423–427
Zobel AM, Hrazdina G (1995) Chalcone synthase localization in early stages of plant development. I. Immunohistochemical use of plasmolysis for localizing the enzyme in epidermal cell cytoplasm of illuminated buckwheat hypocotyls. Biotech Histochem 70:1–6
Zuurbier KW, Leser J, Berger T, Hofte AJ, Schröder G, Verpoorte R, Schröder J (1998) 4-Hydroxy-2-pyrone formation by chalcone and stilbene synthase with nonphysiological substrates. Phytochemistry 49:1945–1951
Acknowledgements
Work in the SAP laboratory is supported by Department of Science and Technology (DST), India, under the INSPIRE Faculty Scheme [DST/INSPIRE/04/2016/001059]. Four anonymous reviewers are acknowledged for their critical reviews and helpful comments that improved the overall quality of the article. Head, Department of Botany is also acknowledged for providing necessary facilities otherwise required for project implementation. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pandith, S.A., Ramazan, S., Khan, M.I. et al. Chalcone synthases (CHSs): the symbolic type III polyketide synthases. Planta 251, 15 (2020). https://doi.org/10.1007/s00425-019-03307-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-019-03307-y