Abstract
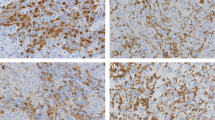
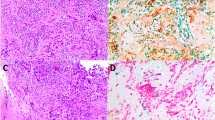
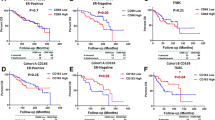
Macrophages are important for the function of the innate immune system, and in solid tumors, they represent a significant proportion of the tumor mass. Tumor-associated macrophages (TAM) have a M2 phenotype and show a multitude of pro-tumoral functions, promoting tumor cell survival, proliferation, and dissemination. CCL2, synthesized by tumor and stromal cells, initiates a chemokine cascade inducing these processes. We studied by immunohistochemistry (IHC) the frequency of TAMs and CCL2 expressing cells in three groups of primary tumor (PT)-recurrence (R) pairs, where relapse was recorded within 2 years (group 1), between 5 and 10 years (group 2), and after 10 years (group 3). In our study all established breast cancers were heavily infiltrated by CD68 positive cells. Both in PTs and in R lesions the infiltration was more abundant in the peritumoral than in the intratumoral stroma. The mean frequency of M2 marker and CD14 positive cells in the intratumoral stroma and CCL2 expressing tumor cells was higher in the Rs as compared to the corresponding PTs. In PTs, a high frequency of CD14 positive cells and a high expression of CCL2 by tumor cells was associated with an early recurrence. The findings support the current understanding of immune cell orchestrated development, progression and metastatic spread of breast cancer. Our study showed that a high frequency of CCL2 positive tumor cells and CD14 positive TAMs are significant risk factors for rapid tumor recurrence. Potential targets for intervention are discussed.



Similar content being viewed by others
References
Gouon-Evans V, Rothenbeg ME, Pollard JW (2000) Postnatal mammary gland development requires macrophages and eosinophils. Development 127:2269–2282
Pollard JW (2009) Trophic macrophages in development and disease. Nat Rev Immunol 9:259–270
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23:549–555
Pollard JW (2004) Tumor-educated macrophages promote tumor progression and metastasis. Nat Rev Cancer 4:71–78
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686
Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RR, Fliser D, Heine GH (2011) SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood 118(12):e50–e61
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969
Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TRDJ, Reedquist KA, Tak PP, Baeten DLP (2012) Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods 375:196–206
Solinas G, Germano G, Mantovani A, Allavena P (2009) Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 86(5):1065–1073
Pollard JW (2008) Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol 84:623–630
Qian B, Li J, Zhang H et al (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475:222–225
Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, van den Bossche J, Mack M, Pipeleers D, in't Veld P, de Baetselier P, van Ginderachter JA (2010) Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 70:5728–5739
Lu X, Kang Y (2009) Chemokine (C-C motif) ligand 2 engages CCR2 + stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J Biol Chem 284:29087–29096
Li M, Knight DA, Snyder LA, Smyth MJ, Stewart TJ (2013) A role for CCL2 in both tumor progression and immunosurveillance. OncoImmunology 2(7):e25474
Zhu X, Fujita L, Snyder A, Okada H (2011) Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. J Neuro-Oncol 104:83–92
Hussein MR, Hassan HI (2006) Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations. J Clin Pathol 59:972–977
Volodko N, Reiner A, Rudas M, Jakesz R (1998) Tumour-associated macrophages in breast cancer and their prognostic correlations. Breast 7:99–105
Medrek C, Ponten F, Jirstrom K, Leandersson K (2012) The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 12:306–315
Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 56:4625–4629
Joensuu K, Leidenius M, Kero M, Andersson LC, Horwitz KB, Heikkilä P (2013) ER, PR, HER2, Ki67 and CK5 in early and late relapsing breast cancer - reduced CK5 expression in metastases. Breast Cancer: Bacic Clin Res 7:23–34
Laoui D, Movahedi K, van Overmeire E, van den Bossche J, Schouppe E, Mommer C, Nikolaou A, Morias Y, de Baetselier P, van Ginderachter JA (2011) Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol 55:861–867
Yang J, Zhang L, Yu C, Yang X-F, Wang H (2014) Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomarker Research 2:1–9
Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, Wojno K, Snyder LA, Yan L, Pienta KJ (2007) Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res 67:9417–9424
Conti I, Rollins BJ (2004) CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol 14:149–154
Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ (2009) CCL2 and Interleukin-6 promote survival of human CD11b + peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem 284(49):34342–34354
Querzoli P, Albonico G, Ferretti S, Rnaldi R, Magri E, Indelli M, Nenci I (1996) MIB-1 proliferative activity in invasive breast cancer measured by image analysis. J Clin Pathol 49(11):926–930
Taneja P, Maglic KF, Zhu S, Kendig RD, Fry EA, Inoue K (2010) Classical and novel prognostic markers for breast cancer and their clinical significance. Clin Med Insights Oncol 4:15–34
Allavena P, Signorelli M, Chiappa M et al (2005) Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): inhibition of macrophage differentiation and cytokine production. Cancer Res 65:2964–2971
Frow EK, Reckless J, Grainger DJ (1999) Tools for anti-inflammatory drug design: in vitro models of leukocyte migration. Med Res Rev 24(3):276–282
Nywening TM, Wang-Gillam A, Sanford DA et al (2016) Phase 1b study targeting tumour associated macrophages with CCR2 inhibition plus FOLFIRINOX in locally advanced and borderline resectable pancreatic cancer. Lancet Oncol 17(5):651–662. https://doi.org/10.1016/S1470-2045(16)00078-4
Giraudo E, Inoue M, Hanahan D (2004) An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest 114:623–633
Kitamura T, Qian B-Z, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li J, Pollard JW (2015) CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med 212(7):1043–1059
Mathenge E, Dean C, Clements D et al (2014) Core needle biopsy of breast Cancer tumors increases distant metastases in a mouse model. Neoplasia 16:950–960
Acknowledgements
We thank Eija Heiliö for her excellent technical assistance, and Antti Nevanlinna, Msc, for facilitating the statistical analysis.
Funding
This work was supported by the Helsinki University Central Hospital Research Foundation, the Finnish Cancer Foundation, the Sigrid Juselius Foundation, and the Finnish Breast Cancer Group.
Author information
Authors and Affiliations
Contributions
Marja Heiskala: doing the literature search and planning the study, evaluating the samples, writing the article, and preparing the graphics
Marjut Leidenius: planning the study, writing the article
Kristiina Joensuu: planning the study, responsible for the collection and handling of the samples, evaluating the immunohistochemical stainings, making the statistical work, and writing the article
Päivi Heikkilä: planning the study and writing the article, analyzing the stainings.
Corresponding author
Ethics declarations
The Ethics Committee of the Helsinki University Central Hospital approved the study protocol.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Heiskala, M., Leidenius, M., Joensuu, K. et al. High expression of CCL2 in tumor cells and abundant infiltration with CD14 positive macrophages predict early relapse in breast cancer. Virchows Arch 474, 3–12 (2019). https://doi.org/10.1007/s00428-018-2461-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-018-2461-7