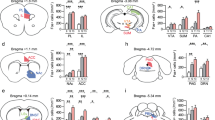
Abstract
Social behavior and stress responses both rely on activity in the prefrontal cortex (PFC) and amygdala. We previously reported that acute stress exposure impoverishes social repertoire and triggers behavioral rigidity, and that both effects are modulated by β2-containing nicotinic receptors. We, therefore, hypothesized that the activity of brain regions associated with the integration of social cues will be modulated by stress exposure. We mapped the expression of c-fos protein in all subregions of the PFC and basolateral (BLA) and central (Ce) areas of the amygdala in C57BL/6J (B6) and β2−/− mice. We show altered brain activity and differences in functional connectivity between the two genotypes after stress: the PFC and BLA were hyperactivated in B6 and hypo-activated in β2−/− mice, showing that the impact of stress on brain activity and functional organization depends on the nicotinic system. We also show that the effects of the opportunity to explore a novel environment or interact socially after acute stress depended on genotype: exploration induced only marginal PFC activation in both genotypes relative to stress alone, excluding a major role for β2 receptors in this process. However, social interaction following stress only activated the rostral and caudolateral areas of the PFC in B6 mice, while it induced a kindling of activation in all PFC and amygdalar areas in β2−/− mice. These results indicate that acute stress triggers important PFC-amygdala plasticity, social interaction has a buffering role during stress-induced brain activation, and β2 receptors contribute to the effects of social interaction under stress.






Similar content being viewed by others
References
Adolphs R, Anderson D (2013) Social and emotional neuroscience. Curr Opin Neurobiol 23:291–293
Arnsten AF (2009) Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10:410–422
Avale ME, Chabout J, Pons S, Serreau P, De Chaumont F, Olivo-Marin JC, Bourgeois JP, Maskos U, Changeux JP, Granon S (2011) Prefrontal nicotinic receptors control novel social interaction between mice. FASEB J 25:2145–2155
Ballesteros-Yáñez I, Benavides-Piccione R, Bourgeois JP, Changeux JP, DeFelipe J (2010) Alterations of cortical pyramidal neurons in mice lacking high-affinity nicotinic receptors. Proc Natl Acad Sci USA 107:11567–11572
Beerling W, Koolhaas JM, Ahnaou A, Bouwknecht JA, de Boer SF, Meerlo P, Drinkenburg WH (2011) Physiological and hormonal responses to novelty exposure in rats are mainly related to ongoing behavioral activity. Physiol Behav 103:412–420
Bicks LK, Koike H, Akbarian S, Morishita H (2015)) Prefrontal cortex and social cognition in mouse and man. Front Psychol 6:1805
Bland ST, Schmid MJ, Der-Avakian A, Watkins LR, Spencer RL, Maier SF (2005) Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res 1051:90–99
Bourgeois JP, Meas-Yeadid V, Lesourd AM, Faure P, Pons S, Maskos U, Changeux JP, Olivo-Marin JC, Granon S (2012) Modulation of the mouse prefrontal cortex activation by neuronal nicotinic receptors during novelty exploration but not by exploration of a familiar environment. Cereb Cortex 22:1007–1015
Buijs RM, Van Eden CG (2000) The integration of stress by the hypothalamus, amygdala and prefrontal cortex: balance between the autonomic nervous system and the neuroendocrine system. Prog Brain Res 126:117–132
Cerqueira JJ, Almeida OF, Sousa N (2008) The stressed prefrontal cortex. Left? Right! Brain. Behavior Immunity 22:630–638
Chowdhury GM, Fujioka T, Nakamura S (2000) Induction and adaptation of Fos expression in the rat brain by two types of acute restraint stress. Brain Res Bull 52:171–182
Coura RS, Cressant A, Xia J, de Chaumont F, Olivo-Marin JC, Pelloux Y, Dalley JW, Granon S (2013) Nonaggressive and adapted social cognition is controlled by the interplay between noradrenergic and nicotinic receptor mechanisms in the prefrontal cortex. FASEB J 27:4343–4354
Craig AD (2010) The sentient self. Brain Struct Funct 214:563–577
Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ (1995) Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64:477–505
Curtis AL, Bello NT, Connolly KR, Valentino RJ (2002) Corticotropin-releasing factor neurones of the central nucleus of the amygdala mediate locus coeruleus activation by cardiovascular stress. J Neuroendocrinol 14:667–682
Dalgleish T (2004) The emotional brain. Nat Rev Neurosci 5:583–589
Day HE, Nebel S, Sasse S, Campeau S (2005) Inhibition of the central extended amygdala by loud noise and restraint stress. Eur J Neurosci 21:441–454
Dayas CV, Buller KM, Crane JW, Xu Y, Day TA (2001) Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci 14:1143–1152
de Andrade JS, Abrão RO, Céspedes IC, Garcia MC, Nascimento JO, Spadari-Bratfisch RC, Melo LL, da Silva RC, Viana MB (2012) Acute restraint differently alters defensive responses and fos immunoreactivity in the rat brain. Behav Brain Res 232:20–29
de Chaumont F, Coura RD, Serreau P, Cressant A, Chabout J, Granon S, Olivo-Marin JC (2012) Computerized video analysis of social interactions in mice. Nat Methods 9:410–417
Del Arco A, Mora F (2009) Neurotransmitters and prefrontal cortex-limbic system interactions implications for plasticity and psychiatric disorders. J Neural Transm 116:941–952
Demolliens M, Isbaine F, Takerkart S, Huguet P, Boussaoud D (2017) Social and asocial prefrontal cortex neurons: a new look at social facilitation and the social brain. Soc Cogn Affect Neurosci 12:1241–1248
dos Santos Coura R, Granon S (2012) Prefrontal neuromodulation by nicotinic receptors for cognitive processes. Psychopharmacology 221:1–18
Douglas LA, Varlinskaya EI, Spear LP (2004) Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol 45:153–162
Dragunow M, Faull R (1989) The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods 29:261–265
Faure A, Pittaras E, Nosjean A, Chabout J, Cressant A, Granon S (2017) Social behaviors and acoustic vocalizations in different strains of mice. Behav Brain Res 1:320:383–390
Fowler CD, Arends MA, Kenny PJ (2008) Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav Pharmacol 19:461-484
Gabbott PL, Warner TA, Jays PR, Bacon SJ (2003) Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res 993:59–71
Gallagher M, Chiba AA (1996) The amygdala and emotion. Cur Opin Neurobiol 6:221–227
Giovannini MG, Rakovska A, Benton RS, Pazzagli M, Bianchi L, Pepeu G (2001) Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience 106:43–53
Goebel M, Stengel A, Wang L, Taché Y (2009) Restraint stress activates nesfatin-1-immunoreactive brain nuclei in rats. Brain Res 1300:114–124
Granon S, Faure P, Changeux JP (2003) Executive and social behaviors under nicotinic receptor regulation. Proc Natl Acad Sci USA 100:9596–9601
Gu X, Liu X, Van Dam NT, Hof PR, Fan J (2013) Cognition-emotion integration in the anterior insular cortex. Cereb Cortex 23:20–27
Heidbreder CA, Groenewegen HJ (2003) The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27:555–579
Herman JP, Cullinan WE (1997) Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20:78–84
Hogg RC, Raggenbass M, Bertrand D (2003) Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol 147:1–46
Holmes A, Wellman CL (2009) Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev 33:773–783
Honkaniemi J, Fuxe K, Rechard L, Koistinaho J, Isola J, Gustafsson J, Okret S, Pelto-Huikko M (1992) Colocalization of fos- and glucocorticoid receptor-like immunoreactivities in the rat amygdaloid complex after immobilization stress. J Neuroendocrinol 4:547–555
Hoover WB, Vertes RP (2007) Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212:149–179
Hurtubise JL, Howland JG (2017) Effects of stress on behavioral flexibility in rodents. Neuroscience 345:176–192
Insel TR, Fernald RD (2004) How the brain processes social information: searching for the social brain. Annu Rev Neurosci 27:697–722
Ito A, Miyoshi M, Ueki S, Fukada M, Komaki R, Watanabe T (2009) “Green odor” inhalation by rats down-regulates stress-induced increases in Fos expression in stress-related forebrain regions. Neurosci Res 65:166–174
Janak PH, Tye KM (2015) From circuits to behaviour in the amygdala. Nature 517:284–292
Jolkkonen E, Pitkänen A (1998) Intrinsic connections of the rat amygdaloid complex: projections originating in the central nucleus. J Comp Neurol 395:53–72
König M, Thinnes A, Klein J (2017) Microdialysis and its use in behavioural studies: Focus on acetylcholine. J Neurosci Methods S0165-0270(17):30294–30297
Kovács KJ (1998) c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int 33:287–297
Kovács KJ (2008) Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol 20:665–672
Krach S, Paulus FM, Bodden M, Kircher T (2010) The rewarding nature of social interactions. Front Behav Neurosci 4:22
Lamm C, Singer T (2010) The role of anterior insular cortex in social emotions. Brain Struct Funct 214:579–591
Lampl I, Katz Y (2017) Neuronal adaptation in the somatosensory system of rodents. Neuroscience 343:66–76
Larrieu T, Sandi C (2018) Stress-induced depression: is social rank a predictive Risk Factor? Bioessays 40:e1800012
Levin ED (2013) Complex relationships of nicotinic receptor actions and cognitive functions. Biochem Pharmacol 86:1145–1152
Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445
Maier SF, Watkins LR (2010) Role of the medial prefrontal cortex in coping and resilience. Brain Res 1355:52–60
McDonald AJ (1991) Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience 44:1–14
McEwen BS, Nasca C, Gray JD (2016) Stress Effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41:3–23
McKlveen JM, Myers B, Herman JP (2015) The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. J Neuroendocrinol 27:446–456
Medford N, Critchley HD (2010) Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct 214:535–549
Muigg P, Scheiber S, Salchner P, Bunck M, Landgraf R, Singewald N (2009) Differential stress-induced neuronal activation patterns in mouse lines selectively bred for high, normal or low anxiety. PLoS One 4:e5346
Murray EA (2007) The amygdala, reward and emotion. Trends Cogn Sci 11:489–497
Nagai M, Kishi K, Kato S (2007) Insular cortex and neuropsychiatric disorders: a review of recent literature. Eur Psychiatry 22:387–394
Nieuwenhuys R (2012) The insular cortex: a review. Prog Brain Res 195:123–163
Nosjean A, Callera JC, Bonagamba L, Machado B, Hamon M, Laguzzi R (2002) Serotonin(3) receptor stimulation in the nucleus tractus solitarii activates non-catecholaminergic neurons in the rat ventrolateral medulla. Neuroscience 112:935–949
Nosjean A, Cressant A, de Chaumont F, Olivo-Marin JC, Chauveau F, Granon S (2015) Acute stress in adulthood impoverishes social choices and triggers aggressiveness in preclinical models. Front Behav Neurosci 8:447
O’Mahony CM, Sweeney FF, Daly E, Dinan TG, Cryan JF (2010) Restraint stress-induced brain activation patterns in two strains of mice differing in their anxiety behaviour. Behav Brain Res 213:148–154
Pacák K, Palkovits M (2001) Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 22:502–548
Pace TW, Gaylord R, Topczewski F, Girotti M, Rubin B, Spencer RL (2005) Immediate-early gene induction in hippocampus and cortex as a result of novel experience is not directly related to the stressfulness of that experience. Eur J Neurosci 22:1679–1690
Patel S, Roelke CT, Rademacher DJ, Hillard CJ (2005) Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signaling. Eur J Neurosci 21:1057–1069
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates, 2nd edn. N.Y. Elsevier Academic Press, New York
Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, Vincent P, Pich EM, Brulet P, Changeux JP (1995) Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature 374:65–67
Picciotto MR, Higley MJ, Mineur YS (2012) Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76:116–129
Picciotto MR, Lewis AS, van Schalkwyk GI, Mineur YS (2015) Mood and anxiety regulation by nicotinic acetylcholine receptors: a potential pathway to modulate aggression and related behavioral states. Neuropharmacology 96:235–243
Pitkänen A, Savander V, LeDoux JE (1997) Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 20:517–523
Pitkänen A, Savander M, Nurminen N, Ylinen A (2003) Intrinsic synaptic circuitry of the amygdala. Ann N Y Acad Sci 985:34–49
Pittaras EC, Faure A, Leray X, Moraitopoulou E, Cressant A, Rabat AA, Meunier C, Fossier P, Granon S (2016) Neuronal nicotinic receptors are crucial for tuning of E/I balance in prelimbic cortex and for decision-making processes. Front Psychiatry 7:171
Poorthuis RB, Goriounova NA, Couey JJ, Mansvelder HD (2009) Nicotinic actions on neuronal networks for cognition: general principles and long-term consequences. Biochem Pharmacol 78:668–676
Potasiewicz A, Hołuj M, Kos T, Popik P, Arias HR, Nikiforuk A (2017) 3-Furan-2-yl-N-p-tolyl-acrylamide, a positive allosteric modulator of the α7 nicotinic receptor, reverses schizophrenia-like cognitive and social deficits in rats. Neuropharmacology 113:188–197
Rasia-Filho AA, Londero RG, Achaval M (2000) Functional activities of the amygdala: an overview. J Psychiatry Neurosci 25:14–23
Reep RL, Winans SS (1982a) Afferent connections of dorsal and ventral agranular insular cortex in the hamster Mesocricetus auratus. Neuroscience 7:1265–1288
Reep RL, Winans SS (1982b) Efferent connections of dorsal and ventral agranular insular cortex in the hamster, Mesocricetus auratus. Neuroscience 7:2609–2635
Robbins TW, Roberts AC (2007) Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb Cortex 17:i151–i160
Roozendaal B, Koolhaas JM, Bohus B (1991) Central amygdala lesions affect behavioral and autonomic balance during stress in rats. Physiol Behav 50:777–781
Sah P, Faber ES, Lopez De Armentia M, Power J (2003) The amygdaloid complex: anatomy and physiology. Physiol Rev 83:803–834
Saha S (2005) Role of the central nucleus of the amygdala in the control of blood pressure: descending pathways to medullary cardiovascular nuclei. Clin Exp Pharmacol Physiol 32:450–456
Salas R, Fung B, Sturm R, De Biasi M (2013) Abnormal social behavior in nicotinic acetylcholine receptor β4 subunit-null mice. Nicotine Tob Res 15:983–986
Senba E, Ueyama T (1997) Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat. Neurosci Res 29:183–207
Shah AA, Treit D (2003) Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res 969:183–194
Shiba Y, Oikonomidis L, Sawiak S, Fryer TD, Hong YT, Cockcroft G, Santangelo AM, Roberts AC (2017) Converging prefronto-insula-amygdala pathways in negative emotion regulation in marmoset monkeys. Biol Psychiatry 82:895–903
Showers C, Cantor N (1985) Social cognition: a look at motivated strategies. Ann Rev Psychol 36:275–305
Valk SL, Bernhardt BC, Trautwein FM, Böckler A, Kanske P, Guizard N, Collins DL, Singer T (2017) Structural plasticity of the social brain: differential change after socio-affective and cognitive mental training. Sci Adv 3:e1700489
Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58
Vertes RP (2006) Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142:1–20
Viau V, Sawchenko PE (2002) Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress: rapid publication. J Comp Neurol 445:293–307
Wallis CU, Cardinal RN, Alexander L, Roberts AC, Clarke HF (2017) Opposing roles of primate areas 25 and 32 and their putative rodent homologs in the regulation of negative emotion. Proc Natl Acad Sci USA 114:E4075–E4084
Wei J, Zhong P, Qin L, Tan T, Yan Z (2017) Chemicogenetic restoration of the prefrontal cortex to amygdala pathway ameliorates stress-induced deficits. Cereb Cortex 11:1–11
Weinberg MS, Girotti M, Spencer RL (2007) Restraint-induced fra-2 and c-fos expression in the rat forebrain: relationship to stress duration. Neuroscience 150:478–486
Yokoyama C, Sasaki K (1999) Regional expressions of Fos-like immunoreactivity in rat cerebral cortex after stress; restraint and intraperitoneal lipopolysaccharide. Brain Res 816:267–275
Acknowledgements
This work was supported by the Centre National de la Recherche Scientifique (CNRS, UMR 9197), by the ANR-FLEXNEURIM and ANR-10-INBS-04 FranceBioImaging Grants. We thank Layla Veleanu for help with some behavioral and immunohistochemical experiments.
Funding
This work was supported by the Centre National de la Recherche Scientifique (CNRS, UMR 9197), by the ANR (FLEXNEURIM 09-BLAN-034002) and ANR (10-INBS-04 FranceBioImaging).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
This article does not contain any studies with human participants performed by any of the authors. This research involves only animals.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. All experimental procedures were carried out in accordance with the EU Directive 2010/63/EU, Decree N 2013-118 of February 1st, 2013, and the French National Committee (87/848).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
. The number (top) and the total time (bottom) of social events statistically correlated with brain area activation in B6 or β2-/- dyads (see Figure 6 and Supplementary Table 4) during the 8 minute social interaction task. Only host mice were submitted to acute stress. Data are expressed as means ± SEM. Histograms show similar quantitative data in the two genotypes. (TIF 2686 KB)
Supplementary Fig. 2
. Plasma corticosterone levels (µg/dl) in different experimental conditions in B6 and β2-/- mice (stressed or not). Blood samples were collected in 3 different experimental conditions: A. 60 min after exit from animal facility. B. after the 45 min of restraint stress. C. after the exploration period. Values are means ± SEM. All comparisons were performed using Kruskal-Wallis one-way ANOVA followed by Mann-Whitney U-tests. Effect of exploration in not stressed mice: *p = 0.01; **p = 0.006. Effect of stress: †† p = 0.01. Effects of stress and exploration: ‡ ‡ p ≤ 0.007. Effect of exploration in stressed mice §p = 0.029. For each experimental procedure, the genotype effect was not significant (0.132 ≤ ps ≤ 0.686). Not Stress mice: n = 4 for each genotype. Stressed mice: n = 6 for each genotype. Mice submitted to exploration with no stress: n = 6 (B6) and n = 7 (β2-/-). Mice submitted to exploration and stress: n = 7 (B6) and n = 9 (β2-/-). (TIF 2876 KB)
Supplementary Table 1
. Statistical comparison between B6 and β2-/- mice with their respective controls (S vs Cnt) and between genotypes (β2-/- vs B6) in not stressed mice (Cnt) and in mice submitted to acute stress (S). Comparisons were performed on 4 independent groups with Kruskal-Wallis one-way ANOVA on Ranks (p) in the Global PFC and in the amygdala (a) and in the rostral, median and caudal PFC (b). When normality passed, data were subsequently analyzed with the Holm-Sidak method test (HS) and when data did not follow a Gaussian distribution it was tested with Mann-Whitney U-tests (MW). p of each test is indicated. Gray areas showed not significant comparisons. (DOCX 23 KB)
Supplementary Table 2
. Statistical analyses in B6 and β2-/- mice submitted to stress with no behavioral procedure (S), to stress followed by exploration of a novel environment (SE), and to stress followed by exploration and social interaction (SESI). Comparisons were performed on six independent groups using Kruskal-Wallis one-way ANOVA on Ranks (p) in the Global PFC and in the amygdala (a) and in the rostral, median and caudal PFC (b). When normality passed, data were subsequently analyzed with the Holm-Sidak method test (HS) and when data did not follow a Gaussian distribution it was tested with Mann-Whitney U-tests (MW). p of each test is indicated. Gray areas showed not significant comparisons. (DOCX 24 KB)
Supplementary Table 3
. Statistical analyses between B6 and β2-/- mice submitted to stress with no behavioral procedure (S), to stress followed by exploration of a novel environment (SE), and to stress followed by exploration of a novel exploration and social interaction (SESI). Statistical analyses were performed on six independent groups using Kruskal-Wallis one-way ANOVA on Ranks (p) in the Global PFC and in the amygdala (a) and in the rostral, median and caudal PFC (b). When normality passed, data were subsequently analyzed with the Holm-Sidak method test (HS) and when data did not follow a Gaussian distribution it was tested with Mann-Whitney U-tests (MW). p of each test is indicated. Gray areas showed not significant comparisons. (DOCX 22 KB)
Supplementary Table 4
. Social behaviors correlated with c-fos protein expression in specific brain areas. Categories and sub-categories of behavioral events during social interaction between an isolated host mouse (IH, black) and a social visitor mouse (SV, white) are extracted from MiceProfiler software (de Chaumont et al. 2012). Only events that correlated with brain structures (see Fig. 6) are represented, i.e., 12 events over the 27 events analyzed. These events are: contact events (close contacts, oral-genital and side-by-side contacts, categories 1 to 3), relative position events (defined by the position of one mouse with regard to the other one, SV mouse is behind IH mouse, category 4), dynamic events initiated by the SV mouse (categories 5 to 7) or by the IH mouse (categories 8 to 11) and IH stop events (category 12). Aggressiveness defined by cumulated numbers of tail rattling exhibited by IH mice and battles defined by cumulated numbers of fight between IH and SV mice, as well as dominance were also analyzed. (DOCX 56 KB)
Rights and permissions
About this article
Cite this article
Nosjean, A., de Chaumont, F., Olivo-Marin, JC. et al. Stress-induced brain activation: buffering role of social behavior and neuronal nicotinic receptors. Brain Struct Funct 223, 4259–4274 (2018). https://doi.org/10.1007/s00429-018-1745-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-018-1745-7