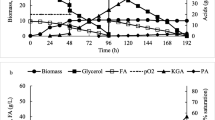
Abstract
The bioconversion process of bioactive naringenin by whole-cells of Yarrowia lipolytica 2.2ab for the production of increased value-added compounds is successfully achieved in surface and liquid cultures. This approach is an alternative to the commercial production of these bioactive compounds from vegetable sources, which are limited due to their low concentrations and the complexity of the purification processes. The experimentation rendered seven value-added compounds in both surface and liquid bioconversion cultures. Some of the compounds produced have not been previously reported as products from the bioconversion processes, such as the case of ampelopsin. Biosynthetic pathways were suggested for the naringenin bioconversion using whole-cells of Y. lipolytica 2.2ab. Finally, the extracts obtained from the naringenin bioconversion in liquid cultures showed higher percentage of inhibition of DPPH· and ABTS· radicals up to 32.88 and 2.08 times, respectively, compared to commercial naringenin.



Similar content being viewed by others
References
Álvarez E, Cambeiro O (2003) Actividad biológica de los flavonoides (I). Acción frente al cáncer Offarm 22:130–140
Karabin M, Hudcova T, Jelinek L, Dostalek P (2014) Biotransformations and biological activities of hop flavonoids. Biotechnol Adv 33:1063–1090. https://doi.org/10.1016/j.biotechadv.2015.02.009
Ribeiro IA, Rocha J, Sepodes B, Mota-Filipe H, Ribeiro MH (2008) Effect of naringin enzymatic hydrolysis towards naringenin on the anti-inflammatory activity of both compounds. J Mol Catal B Enzym 52–53:13–18. https://doi.org/10.1016/j.molcatb.2007.10.011
Olsen KM, Hehn A, Jugdé H, Slimestad R, Larbat R, Bourgaud F, Lillo C (2010) Identification and characterisation of CYP75A31, a new flavonoid 3’5’-hydroxylase, isolated from Solanum lycopersicum. BMC Plant Biol 10:1–12. https://doi.org/10.1186/1471-2229-10-21
Markovic JD (2007) Flavonoids, the role and the importance in modern investigations. Acta Agric Serb XII:25–36
Madej A, Popłoński J, Huszcza E (2014) Improved oxidation of naringenin to carthamidin and isocarthamidin by Rhodotorula marina. Appl Biochem Biotechnol 173:67–73. https://doi.org/10.1007/s12010-014-0787-4
Das S, Rosazza JPN (2006) Microbial and enzymatic transformations of flavonoids. J Nat Prodvol 69:499–508. https://doi.org/10.1021/np0504659
Zhang J (2007) Flavonoids in grapefruit and commercial grapefruit juices: concentration, distribution, and potential health benefits. Proc Fla State Hort Soc 120:288–294
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747. https://doi.org/10.1093/ajcn/79.5.727
Tong X, Smith KA, Pelling JC (2012) Apigenin, a chemopreventive bioflavonoid, induces AMP-activated protein kinase activation in human keratinocytes. Mol Carcinog 51:268–279. https://doi.org/10.1002/mc.20793
López-Lázaro M (2009) Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem 9:31–59. https://doi.org/10.2174/138955709787001712
Marín L, Gutiérrez-del-Río I, Entrialgo-Cadierno R, Villar CJ, Lombó F (2018) De novo biosynthesis of myricetin, kaempferol and quercetin in Streptomyces albus and Streptomyces coelicolor. PLoS ONE 13:1–16. https://doi.org/10.1371/journal.pone.0207278
Pinto JT, Alvarenga LF, Pinto de Oliveira D, Toledo de Oliveira T, Schwan RF, Dias DR, de Queiroz JH (2017) Elaboration and characterization of Japanese Raisin Tree (Hovenia dulcis Thumb.) pseudofruits fermented alcoholic beverage. Food Sci Technol 37:101–108. https://doi.org/10.1590/1678-457x.25616
Cheng P, Gui C, Huang J, Xia Y, Fang Y, Da G, Zhang X (2017) Molecular mechanisms of ampelopsin from Ampelopsis megalophylla induces apoptosis in HeLa cells. Oncol Lett 14:2691–2698. https://doi.org/10.3892/ol.2017.6520
Kou X, Chen N (2012) Pharmacological potential of ampelopsin in Rattan tea. Food Sci Hum Wellness 1:14–18. https://doi.org/10.1016/j.fshw.2012.08.001
Kou X, Fan J, Chen N (2017) Potential molecular targets of ampelopsin in prevention and treatment of cancers. Anticancer Agents Med Chem 17:1610–1616. https://doi.org/10.2174/1871521409666170412130529
Jiménez-Atiénzar M, Escribano J, Cabanes J, Gandía-Herrero F, García-Carmona F (2005) Oxidation of the flavonoid eriodictyol by tyrosinase. Plant Physiol Biochem 43:866–873. https://doi.org/10.1016/j.plaphy.2005.07.010
Minato KI, Miyake Y, Fukumoto S, Yamamoto K, Kato Y, Shimomura Y, Osawa T (2003) Lemon flavonoid, eriocitrin, suppresses exercise-induced oxidative damage in rat liver. Life Sci 72(14):1609–1616. https://doi.org/10.1016/S0024-3205(02)02443-8
Xu J, Yang L, Zhao SJ, Wang ZT, Hu ZB (2012) An efficient way from naringenin to carthamidine and isocarthamidine by Aspergillus niger. World J Microbiol Biotechnol 28:1803–1806. https://doi.org/10.1007/s11274-011-0934-9
Nagy TO, Ledolter K, Solar S (2008) Oxidation of naringenin by gamma-radiation. Radiat Phys Chem 77:728–733. https://doi.org/10.1016/j.radphyschem.2007.10.007
Kasai N, Ikushiro S-i, Hirosue S, Arisawa A, Ichinose H, Wariishi H, Ohta M, Sakaki T (2009) Enzymatic properties of cytochrome P450 catalyzing 3´-hydroxylation of naringenin from the white-rot fungus Phanerochaete chrysosporium. Biochem Biophys Res Commun 387:103–108. https://doi.org/10.1016/j.bbrc.2009.06.134
Ribeiro IA, Ribeiro MHL (2008) Naringin and naringenin determination and control in grapefruit juice by a validated HPLC method. Food Control 19:432–438. https://doi.org/10.1016/j.foodcont.2007.05.007
Prasetyo EN, Nyanhongo GS, Guebitz GM (2011) Enzymatically enriching naringenin with hydroxylated and/or methoxylated phenolic compounds. Process Biochem 46:1019–1024. https://doi.org/10.1016/j.procbio.2010.12.017
Crozier A, Jaganath IB, Clifford MN (2009) Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep 26:1001–1043. https://doi.org/10.1039/b802662a
Cao H, Chen X, Jassbi AR, Xiao J (2015) Microbial biotransformation of bioactive flavonoids. Biotechnol Adv 33:214–223. https://doi.org/10.1016/j.biotechadv.2014.10.012
Lee JH, Oh ET, Chun SC, Keum YS (2014) Biotransformation of isoflavones by Aspergillus niger and Cunninghamella elegans. J Korean Soc Appl Biol Chem 57:523–527. https://doi.org/10.1007/s13765-014-4145-6
Velasco RB, Montenegro DLM, Vélez JFS, García CMP, Durango DLR (2009) Biotransformación de compuestos aromáticos sustituidos mediante hongos filamentosos fitopatógenos de los géneros botryodiplodia y colletotrichum. Rev Soc Quím Perú 75:94–111
Hirakawa K, Kobayashi S, Inoue T, Endoh-Yamagami S, Fukuda R, Ohta A (2009) Yas3p, an opi1 family transcription factor, regulates Cytochrome P450 expression in response to n-alkanes in Yarrowia lipolytica. J Biol Chem 284:7126–7137. https://doi.org/10.1074/jbc.M806864200
Holland HL, Weber HK (2000) Enzymatic hydroxylation reactions. Curr Opin Biotechnol 11:547–553. https://doi.org/10.1016/S0958-1669(00)00142-7
Chu LL, Pandey RP, Jung N, Jung HJ, Kim E-H, Sohng JK (2016) Hydroxylation of diverse flavonoids by CYP450 BM3 variants: biosynthesis of eriodictyol from naringenin in whole cells and its biological activities. Microb Cell Fact 15:1–15. https://doi.org/10.1186/s12934-016-0533-4
Amor IL-B, Salem N, Guedon E, Engasser J-M, Chekir-Ghedrira L, Ghoul M (2010) Preliminary investigation of naringenin hydroxylation with recombinant E. coli expressing plant flavonoid hydroxylation gene. Nat Prod Commun 5:777–782
Chang T-S, Lin M-Y, Lin H-J (2010) Identifying 8-hydroxynaringenin as a suicide substrate of mushroom tyrosinase. J Cosmet Sci 61:205–210. https://doi.org/10.1111/j.1468-2494.2010.00619_1.x
Coelho MAZ, Amaral PFF, Belo I (2010) Yarrowia lipolytica: an industrial workhorse. Appl Microbiol Microb Biotechnol 1:930–944
Palmerin-Carreño DM, Rutiaga-Quiñones OM, Verde Calvo JR, Prado-Barragan A, Huerta-Ochoa S (2015) Screening of microorganisms for bioconversion of (+)-valencene to (+)-nootkatone. LWT Food Sci Technol 64:788–793. https://doi.org/10.1016/j.lwt.2015.06.065
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Kim DO, Chun OK, Kim YJ, Moon HY, Lee CY (2003) Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem 51:6509–6515. https://doi.org/10.1021/jf0343074
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559. https://doi.org/10.1016/S0308-8146(98)00102-2
Pérez-Nájera V, Lugo-Cervantes E, Gutiérrez-Lomelí M, Del-Toro-Sánchez C (2013) Extracción de compuestos fenólicos de la cáscara de lima (citrus limetta risso) y determinación de su actividad antioxidante. Rev Ciencias Biol La Salud XV:1665–1456. https://doi.org/10.18633/bt.v15i3.153
Fajardo-Romero A, Arroyo-Rivera Á, Ramírez-Navas JS (2017) Extracción de flavonoides totales de la envoltura externa de cebolla roja (Allium cepa). UGCiencia 22:119–126. https://doi.org/10.18634/ugcj.22v.1i.599
Marín L, Gutiérrez-del-Río I, Yagüe P, Manteca Á, Villar CJ, Lombó F (2017) De novo biosynthesis of apigenin, luteolin, and eriodictyol in the actinomycete Streptomyces albus and production improvement by feeding and spore conditioning. Front Microbiol 8:1–12. https://doi.org/10.3389/fmicb.2017.00921
Park SR, Paik JH, Ahn MS, Park JW, Yoon YJ (2010) Biosynthesis of plant-specific flavones and flavonols in Streptomyces venezuelae. J Microbiol Biotechnol 20:1295–1299. https://doi.org/10.4014/jmb.1005.05038
Park SR, Ahn MS, Han AR, Park JW, Yoon YJ (2011) Enhanced flavonoid production in Streptomyces venezuelae via metabolic engineering. J Microbiol Biotechnol 21:1143–1146. https://doi.org/10.4014/jmb.1108.08012
Chung DM, Chung YC, Maeng PJ, Chun HK (2011) Regioselective deglycosylation of onion quercetin glucosides by Saccharomyces cerevisiae. Biotechnol Lett 33:783–786. https://doi.org/10.1007/s10529-010-0501-8
Zhu S, Wu J, Du G, Zhou J, Chen J (2003) Efficient synthesis of eriodictyol from l-tyrosine in escherichia coli. Appl Environ Microbiol 80:3072–3080. https://doi.org/10.1128/AEM.03986-13
Trueba GP (2003) Los flavonoides: antioxidantes o prooxidantes: Rev Cuba. Investig Biomed 22:48–57
Leonard E, Yan Y, Koffas MAG (2006) Functional expression of a P450 flavonoid hydroxylase for the biosynthesis of plant-specific hydroxylated flavonols in Escherichia coli. Metab Eng 8:172–181. https://doi.org/10.1016/j.ymben.2005.11.001
Trantas E, Panopoulos N, Ververidis F (2009) Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae. Metab Eng 6:355–366. https://doi.org/10.1016/j.ymben.2009.07.004
Semwal DK, Semwal RB, Combrinck S, Viljoen A (2016) Myricetin: a dietary molecule with diverse biological activities. Nutrients 8:1–31. https://doi.org/10.3390/nu8020090
González-Mendoza D (2007) El complejo enzimático citocromo P450 en las plantas. Rev Int Contam Ambient 23:177–183
Araújo WL, Martins AO, Fernie AR, Tohge T (2014) “2-Oxoglutarate: linking TCA cycle function with amino acid, glucosinolate, flavonoid, alkaloid, and gibberellin biosynthesis. Front Plant Sci 5:1–6. https://doi.org/10.3389/fpls.2014.00552
Bordewick S, Beier A, Balke K, Bornscheuer UT (2018) Baeyer-Villiger monooxygenases from Yarrowia lipolytica catalyze preferentially sulfoxidations. Enzyme Microb Technol 109:31–42. https://doi.org/10.1016/j.enzmictec.2017.09.008
Osadebe PO, Okoye FBC, Uzor PF, Nnamani NR, Adiele IE, Obiano NC (2012) Phytochemical analysis, hepatoprotective and antioxidant activity of Alchornea cordifolia methanol leaf extract on carbon tetrachloride-induced hepatic damage in rats. Asian Pac J Trop Med 5:289–293. https://doi.org/10.1016/S1995-7645(12)60041-8
Habtemariam S (1997) Flavonoids as inhibitors or enhancers of the cytotoxicity of tumor necrosis factor-α in L-929 tumor cells. J Nat Prod 60:775–778. https://doi.org/10.1021/np960581z
Dangrit D, Sompornpailin K (2018) Antioxidant activities of transgenic flower over-expressing FLS and TT8 involving in flavonoid biosynthesis. Appl Mech Mater 879:78–82. https://doi.org/10.4028/www.scientific.net/AMM.879.78
Frejnagel S (2007) Comparison of polyphenolic composition of extracts from honeysuckle, chokeberries and green tea: a short report. Pol J Food Nutr Sci 57:83–86
Bondet V, Brand-Williams W, Berset C (1997) Kinetics and mechanisms of antioxidant activity using the DPPH. free radical method. LWT - Food Sci Technol 30:609–615. https://doi.org/10.1006/fstl.1997.0240
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic compounds. Free Radical Bio Med 20(7):933–956. https://doi.org/10.1016/0891-5849(95)02227-9
Acknowledgments
We are grateful to the National Council of Science and Technology (Conacyt México) for financial support for project SEP-CONACyT-2013-CO1-220867, and through a Christian Hernández Guzmán PhD Grant 302006.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hernández-Guzmán, C., Prado-Barragán, A., Gimeno, M. et al. Whole-cell bioconversion of naringenin to high added value hydroxylated compounds using Yarrowia lipolytica 2.2ab in surface and liquid cultures. Bioprocess Biosyst Eng 43, 1219–1230 (2020). https://doi.org/10.1007/s00449-020-02316-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02316-6