Abstract
Background
Esophageal perforations and postoperative leakage of esophagogastrostomies are considered to be life-threatening conditions due to the potential development of mediastinitis and consecutive sepsis. Vacuum-assisted closure (VAC) techniques, a well-established treatment method for superficial infected wounds, are based on a negative pressure applied to the wound via a vacuum-sealed sponge. Endoluminal VAC (E-VAC) therapy as a treatment for GI leakages in the rectum was introduced in 2008. E-VAC therapy is a novel method, and experience regarding esophageal applications is limited. In this retrospective study, the experience of a high-volume center for upper GI surgery with E-VAC therapy in patients with leaks of the upper GI tract is summarized. To our knowledge, this series presents the largest patient cohort worldwide in a single-center study.
Methods
Between October 2010 and January 2017, 77 patients with defects in the upper gastrointestinal tract were treated using the E-VAC application. Six patients had a spontaneous perforation, 12 patients an iatrogenic injury, and 59 patients a postoperative leakage in the upper gastrointestinal tract.
Results
Complete restoration of the esophageal defect was achieved in 60 of 77 patients. The average duration of application was 11.0 days, and a median of 2.75 E-VAC systems were used. For 21 of the 77 patients, E-VAC therapy was combined with the placement of self-expanding metal stents.
Conclusion
This study demonstrates that E-VAC therapy provides an additional treatment option for esophageal wall defects. Esophageal defects and mediastinal abscesses can be treated with E-VAC therapy where endoscopic stenting may not be possible. A prospective multi-center study has to be directed to bring evidence to the superiority of E-VAC therapy for patients suffering from upper GI defects.
Similar content being viewed by others
Esophageal perforations and postoperative leakage of esophagogastrostomies are considered to be life-threatening conditions due to the potential development of mediastinitis and consecutive sepsis [1].
Vacuum-assisted closure (VAC) techniques, a well-established treatment method for superficial infected wounds, are based on a negative pressure applied to the wound via a vacuum-sealed sponge [2, 3]. Endoluminal VAC (E-VAC) therapy is a novel method and experience regarding esophageal applications is limited.
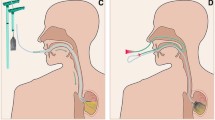
In 2014, we have published our first experience in E-VAC therapy in 14 patients over a time period of 3 years [4]. In this time period, upper GI leakages were treated mainly with endoscopic stenting [5]. Self-expanding metal or plastic stents were usually used for these complications. In cases of a persisting sepsis, the undrained mediastinal abscess formation was often difficult to reach by interventional radiological treatment (Fig. 1). A delayed therapy for more than 24 h causes high morbidity and mortality [1].
E-VAC therapy was used reluctantly, but this changed due to our own promising results of E-VAC therapy. In this retrospective study, the experience of the largest German center of excellence for upper GI surgery with endoluminal VAC therapy in patients with leakages of the upper GI tract is summarized. To our knowledge, this series presents the largest single-center patient’s cohort.
Patients and methods
In this retrospective study, 77 patients (26 women and 51 men) with leakages in the upper GI tract were treated using the E-VAC, other endoscopic treatment options, or conventional surgical therapy between October 2010 and January 2017.
The average age of the patients was 64.1 years (range 37.9–86.6 years). The Ethics Committee of the University of Cologne approved the study (Nr. 13-096). The primary outcome of the study was closure of the leakage. Furthermore, complications and side effects of E-VAC therapy were recorded.
The defects in the upper gastrointestinal tract were divided into three groups: spontaneous perforation, iatrogenic defect, and postoperative leakage in the upper gastrointestinal tract. The group of patients with postoperative leakages was subdivided into a group of anastomotic insufficiencies of the esophagogastrostomy after Ivor-Lewis procedure, a group of insufficiencies of the esophagojejunostomy after gastrectomy, and a group of defects after various surgical procedures (surgical defects).
The first group consists of six patients suffering from Boerhaave’s syndrome. Iatrogenic perforations occurred in 12 patients due to endoscopic (9/12) and diagnostic (3/12) interventions. The third group consists of patients suffering from leakages after upper GI surgery in case of anastomotic insufficiency or surgical preparation. Fifty-nine patients in the third group experienced a leakage after esophagectomy (n = 36), after gastrectomy (n = 15), or after various other surgical procedures (8/59).
In 72 patients, the leakage was diagnosed by endoscopic examination, in two patients by contrast medium swallow, and in 3 patients by computed tomography (CT) scan.
E-VAC treatment
Principle
An open-pore polyurethane foam drainage tube is inserted into the cavity of the leakage (intracavitary) or directly into the lumen (intraluminal) under direct endoscopic view based on the size of the leakage. Subsequently, a continuous therapeutic vacuum of 100–125 mmHg is produced with an electronic pump through the diverted drainage tube. The negative pressure that is built up by the pump is then transmitted evenly to the tissue through the foam (Fig. 2). As a result of the negative pressure, the wound cavity (intracavitary) is cleaned mechanically from microorganisms and the interstitial edema is reduced by improvement of microcirculation. Extraluminal wound closure takes place consecutively as a result of increased granulation and re-epithelialization (Fig. 4). Primary defect closure occurs due to intraluminal placement of the polyurethane foam drainage tube.
Realization
A combined diverted tube-foam design has been proven to be effective in our patient population. A triple-lumen diverted nasal tube (e.g., Freka® Trelumina, Fresenius Kabi Germany GmbH) is inserted into the gastric interponate prior to placement of the polyurethane foam drainage tube (e.g., EsoSponge®, Braun) for this purpose. This ensures enteral feeding throughout the entire duration of therapy.
In the case of suspected perforation or anastomosis insufficiency, flexible endoscopy with an esophagogastroduodenoscope (e.g., GIF-H190, GIF-Q165, GIF-XP190N, Olympus Corporation, Tokyo, Japan) is predominantly performed. If an anastomotic insufficiency is not diagnosed with certainty, endoscopy is repeated at regular intervals in addition to other imaging examinations (e.g., CT scan).
If the leakage/defect can be diagnosed endoscopically, the height of the defect is recorded first by reporting the distance from the incisors. Afterwards, the size of the leakage/defect is measured with the endoscope that is still inside the patient.
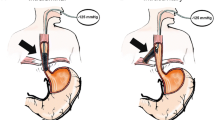
In the case of smaller defects, rinsing (e.g., with 50 ml sterile water) or a nasal endoscope is used to check whether there is an extraluminal wound cavity. If this is not the case, intraluminal (Fig. 3) insertion of the polyurethane foam drainage tube would be the preferred procedure.
In the case of larger-sized leakages in the presence of a wound cavity, intracavitary (Fig. 4) insertion should be favored. Next, open-pore polyurethane foam is adapted individually to the size of the defect. Oral application of the foam into the esophagus is ensured by means of a protective tube (“overtube”) that protects the upper esophageal sphincter from injury and keeps it open at the same time. Subsequently, the individually adapted polyurethane foam drainage tube can be pushed forward to the placement location with a “pusher.” After the drainage tube has been placed, both the pusher and the overtube are removed. Afterwards, the polyurethane foam drainage tube is pushed to the placement location under direct view using an endoscope. In the case of a difficult anatomical situation, foreign body forceps (e.g., Alligatormouth 1-to-2-teeth, MTW Endoskopie W. Haag KG) are used instead to achieve better placement.
Both procedures (intraluminal and intracavitary placement) are demonstrated in a short video.
After verifying correct positioning, the drainage tube is diverted from oral to nasal and then fixed in position in order to prevent the system from dislocating. This is done by means of a strip of adhesive bandage or, optionally, using a nasal retaining system (e.g., AMT Bridle™, Applied Medical Technology). Subsequently, the diverted drainage tube is connected to an electronic vacuum pump (e.g., VivanoTec®, Hartmann AG, Germany) and a defined vacuum of 100–125 mmHg is applied. As a result of continuous negative pressure, the foam drainage tube fixes itself in position preventing dislocation. Elective endoscopic wound control is carried out after 3–4 days. In order to do this, the suction is interrupted and the drainage tube is disconnected. A disconnection between the foam and the surrounding tissue is achieved by rinsing with 30–50 ml sterile water through the disconnected drainage tube and subtile mobilization of the foam. Afterwards, the drainage tube, which has been diverted in an oral direction in the meantime, will be easy to remove by pulling. If the drainage tube has to be placed again, this is done following the steps described above. In this case, the foam drainage tube must be changed regularly, as this prevents the foam from growing into the wound cavity. The duration of treatment with the foam drainage tube depends on the size of the insufficiency and the wound cavity. The therapy should not be terminated until the insufficiency is closed or the wound cavity is fully lined with granulation tissue. Alternatively, the procedure can be changed to SEMS.
Results
In Table 1, the etiology and location of spontaneous and iatrogenic esophageal perforations as well as patients with postoperative leakages are demonstrated.
All patients suffering from Boerhaave’s syndrome had perforations in the distal third of the esophagus.
Iatrogenic perforations occurred in seven patients after balloon dilatation of stenosis, scleroderma, or achalasia. In five patients, different interventional procedures (gastric tube, transesophageal echography, removal of a foreign object, POEM procedure) caused perforations of the proximal (n = 2), middle (n = 2), or distal third (n = 1) of the esophagus.
The main part of upper GI leakages occurred in 59 patients postoperatively. In this group, mainly patients after Ivor-Lewis esophagectomy (n = 36) and after gastrectomy (n = 15) were presented. The smaller group of eight patients had developed postoperative leakages in the upper GI tract because of surgical defects in the proximal (n = 2), the middle (n = 2), distal (n = 3) esophagus or even in the proximal jejunum (n = 1).
These patients were treated endoscopically with E-VAC therapy in a very advanced setting (Table 2). E-VAC therapy was performed in all 77 patients.
In 34 patients, the E-VAC therapy was combined with placement of an endoscopic feeding tube, in 21 patients in combination with the placement of SEMS. The stent was placed after the E-VAC therapy. Removal of the stent was performed in between 5 and 6 weeks afterwards. The closure was confirmed by endoscopy or by CT scan with oral contrast or a contrast swallow before the beginning of oral intake.
In 68 patients, E-VAC system was placed intraluminally and in 12 patients intracavitarily.
The median duration of application was 11.0 days (range 1–65) per patient. A median number of 2.75 (range 1–9) sponges per patient were used with a median interval between changes, 4.0 days (range 2–5) days.
Table 3 summarizes the results of the follow-up and outcome after advanced endoscopic treatment. Mean follow-up of all 77 patients was 165.8 days. Conversion to surgical approach after E-VAC was conducted if a successful treatment with E-VAC therapy was not possible. This was necessary in five patients: one patient with a Boerhaave’s syndrome, three patients after iatrogenic perforations (gastric tube, after balloon dilatation, after transesophageal echography), and one patient after gastrectomy.
Complete healing of the defect was achieved in 60/77 patients (77.9%) cases (Fig. 5). Incomplete closure was seen in four patients, 10 out of 77 patients deceased because of multi-organ failure, severe hemorrhage, or lethal pulmonal embolism.
In the group of spontaneous perforations, 4 of 6 patients (66.7%) with Boerhaave’s syndrome had an effective treatment with E-VAC system. One patient (1/6) died because of multi-organ-failure and 1 patient was converted to surgical therapy.
In the group of iatrogenic perforations, 9 of 12 patients (75%) were treated successfully with the E-VAC system. Three patients had surgical interventions with suturing of the defect and protection with a hemifundoplication; two of these three patients died because of severe postoperative hemorrhage or pulmonal embolism.
In the third group of postoperative leakages in 46 of 59 (77.9%) patients, the leakage healed with the endoscopic technique. Nine patients deceased as a consequence of multi-organ failure (n = 6), pulmonal embolism (n = 1), or severe hemorrhage (n = 2). Three patients with multi-organ failure died due to acute respiratory distress syndrome.
Discussion
Esophageal perforations and postoperative leakages after upper GI surgery are associated with a high rate of morbidity and mortality [1]. Endoscopic treatment of upper GI leakages was mainly conducted of using endoscopic stents for a long time.
E-VAC therapy as a treatment for GI leakages in the rectum was introduced in 2008 [4]. A series of 29 patients was described. Loske and Müller transferred this treatment option to the upper GI tract in 2007 [6, 7].
In the last 10 years, the E-VAC therapy for treatment upper GI defects has become a treatment alternative to endoscopic stenting in numerous German endoscopic centers [6, 8,9,10,11]. This can be demonstrated in published case series of more than 200 patients mainly of working groups in Germany.
In 2015, the first series from Dallas, USA [12] was published, and in 2016 a group from Korea [13] demonstrated their experience with E-VAC therapy. They conducted a retrospective analysis on outcome of postoperative upper GI leaks in 18 patients, who were treated with stents or E-VAC therapy.
In 2017, Kuehn et al. [14] published a review on endoscopic vacuum therapy for various defects of the upper GI tract. Their Medline analysis showed 11 case series with over 210 patients with E-VAC treatment. Overall healing rate was achieved in 180/210 patients (90%). In this review, the success rate for anastomotic leakage was 90%, 107/119 patients, and the success rate for esophageal perforations was achieved in 96%, 49/52. The median duration of therapy took 17 days. The authors concluded that E-VAC therapy is a valuable approach even in various complex situations.
In 2014, we have published our first experience in E-VAC therapy in 14 patients over a time period of 3 years [4]. In this time period, upper GI leakages were treated mainly with endoscopic stenting [5]. E-VAC therapy was reluctantly used, but this changed due to our own promising results of E-VAC therapy. In our learning curve of application, E-VAC therapy establishing a continuous suction with an electronic pump was complicated due to not standardized connection devices.
This retrospective single-center study of prospectively collected data including 77 patients treated with the E-VAC system is to our knowledge the largest series of patients suffering from upper GI leakages.
The results of our study are comparable to the results of the 11 published series including 210 patients. The success rate in our series was 78% in comparison to 90% in all other studies.
The median number of E-VAC treatments and the intervals were comparable to the published literature; however, our series demonstrated a larger portion of combined treatments (E-VAC/stent). With this we achieved a shorter duration of treatment and a reduction of E-VAC changes.
The lower success rate in our collective may be contributed to the negative selection of more severe cases since our institution is the main referral center for upper GI perforations in Germany. In addition, up to 15% of the cohort consists of patients who were transferred to our center in septic circumstances with often delayed diagnosed leakage or perforations. This results, as we have demonstrated, in higher morbidity and mortality [1].
Over 70% of these patients after Ivor-Lewis esophagectomy and gastrectomy have been pretreated with preoperative chemo-radiotherapy or perioperative chemotherapy. Many of these patients are afflicted with malnutrition, which means that in case of anastomotic leakage enteral nutrition should be ensured to improve the healing process. The combination of E-VAC and endoscopic feeding tube was performed in 34 of 77 patients. As described above, we use a triple-lumen diverted nasal tube, which enables enteral feeding in the deep duodenum and at the same time evacuation of gastric exudation in the gastric conduit associated to delayed gastric emptying which is quite common after Ivor-Lewis procedure [11].
In this patient’s series, we demonstrated the successful E-VAC therapy even in advanced cases: one patient suffered from anastomotic leakage in the proximal jejunum 75 cm from the incisors. Any surgical procedure was impossible due to extensive adhesions. Anastomotic leakage was successfully treated with 16 E-VAC systems over a time period of 65 days and abdominal CT-guided drainage. Successful treatment of complex cases with a combination of different techniques (E-VAC, SEMS, CT-guided drainage, endoscopically placed feeding tube) was described in several other reports as well [11, 16,17,18].
In the beginning of interventional endoscopic treatment, we have demonstrated that implantation of covered SEMS in patients with esophageal leak or perforation is a safe and feasible alternative to operative treatment and can lower the interventional morbidity rate [5]. But in some patients, sepsis persists due to an undrained mediastinal abscess formation, which often is difficult to address by interventional radiologic means. This problem of undrained abscess is prevented with E-VAC therapy more easily.
The advantages of interventional E-VAC technique to surgical revisions were shown as well in 3 retrospective studies [11, 19, 20]: patients with septicemia and major esophageal defects had benefits in comparison to patients treated with stent or surgical revisions.
Currently, there are no prospective randomized clinical trials available comparing endoscopic stenting and E-VAC therapy in upper GI leakages.
In review of our improved knowledge with E-VAC therapy, we favor E-VAC therapy as first treatment option for upper GI leakages. We have adapted the algorithm of Schorsch et al. to our treatment processes of anastomotic leakage (Fig. 6) [7].
Further research by registry analysis and prospectively randomized multi-center clinical trials is needed to generate more evidence for the superiority of E-VAC therapy in patients suffering from upper GI leakages.
Conclusion
This study verifies that E-VAC treatment for leakages in upper GI leakages is secure and successful. E-VAC therapy increases the spectrum of interventional endoscopy even under complex circumstances.
The combination of E-VAC therapy with endoscopic feeding tube placement and endoscopic stenting widens current treatment options. E-VAC therapy as an interventional technique pools two surgical treatment strategies for upper GI defects: Draining the mediastinal or pleural abscess and closing the defect of the leakage with the possibility of concurrent enteral feeding.
The encouraging E-VAC technique has reduced the need of surgical revision in many cases. Nevertheless, surgical revision still has its indication in patients with persisting sepsis with the importance of removing the site of primary infection adequately.
References
Vallböhmer D, Hölscher AH, Hölscher M, Bludau M, Gutschow C, Stippel D et al (2010) Options in the management of esophageal perforation: analysis over a 12-year period. Dis Esophagus 23(3):185–190
Fleischmann W, Strecker W, Bombelli M, Kinzl L (1993) Vacuum sealing as treatment of soft tissue damage in open fractures. Unfallchirurg 96(9):488–492
Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS (2005) Mechanisms and clinical applications of the vacuum-assisted closure (VAC) Device: a review. Am J Clin Dermatol 6(3):185–194
Bludau M, Hölscher AH, Herbold T, Leers JM, Gutschow C, Fuchs H et al (2014) Management of upper intestinal leaks using an endoscopic vacuum-assisted closure system (E-VAC). Surg Endosc 28(3):896–901
Leers JM, Vivaldi C, Schäfer H, Bludau M, Brabender J, Lurje G et al (2009) Endoscopic therapy for esophageal perforation or anastomotic leak with a self-expandable metallic stent. Surg Endosc 23(10):2258–2262
Loske G, Schorsch T, Müller C (2011) Intraluminal and intracavitary vacuum therapy for esophageal leakage: a new endoscopic minimally invasive approach. Endoscopy 43(6):540–544
Schorsch T, Müller C, Loske G (2014) Endoscopic vacuum therapy of perforations and anastomotic insufficiency of the esophagus. Chirurg 85(12):1081–1093
Weidenhagen R, Hartl WH, Gruetzner KU, Eichhorn ME, Spelsberg F, Jauch KW (2010) Anastomotic leakage after esophageal resection: new treatment options by endoluminal vacuum therapy. Ann Thorac Surg 90(5):1674–1681
Laukoetter MG, Mennigen R, Neumann PA, Dhayat S, Horst G, Palmes D et al (2016) Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc. doi10.1007/s00464-016-5265-3
Mennigen R, Senninger N, Laukoetter MG (2014) Novel treatment options for perforations of the upper gastrointestinal tract: endoscopic vacuum therapy and over-the-scope clips. World J Gastroenterol 20(24):7767–7776
Schniewind B, Schafmayer C, Voehrs G, Egberts J, von Schoenfels W, Rose T et al (2013) Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc 27(10):3883–3890
Smallwood NR, Fleshman JW, Leeds SG, Burdick JS (2016) The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg Endosc 30(6):2473–2480
Hwang JJ, Jeong YS, Park YS, Yoon H, Shin CM, Kim N et al (2016) Comparison of endoscopic vacuum therapy and endoscopic stent implantation with self-expandable metal stent in treating postsurgical gastroesophageal leakage. Medicine 95(16):e3416
Kuehn F, Loske G, Schiffmann L, Gock M, Klar E (2017) Endoscopic vacuum therapy for various defects of the upper gastrointestinal tract. Surg Endosc. doi:10.1007/s00464-016-5404-x
Maus MKH, Leers J, Herbold T, Bludau M, Chon S-H, Kleinert R et al (2016) Gastric outlet obstruction after esophagectomy: retrospective analysis of the effectiveness and safety of postoperative endoscopic pyloric dilatation. World J Surg 40(10):2405–2411
Loske G, Schorsch T, Schmidt-Seithe H, Müller C (2014) Intraluminal endoscopic vacuum therapy in a case of ischemia of the blind end of the jejunal loop after Roux-en-Y gastrectomy. Endoscopy 46(S 01):E575–E576
Schröder W, Leers JM, Bludau M, Herbold T, Hölscher AH (2014) Kardianahe Perforation bei gutartigen Erkrankungen. Der Chir 85(12):1064–1072
Loske G, Schorsch T, van Ackeren V, Schulze W, Müller CT (2015) Endoscopic vacuum therapy in Boerhaave’s syndrome with open-pore polyurethane foam and a new open-pore film drainage. Endoscopy 47(Suppl 1):E410-1
Brangewitz M, Voigtländer T, Helfritz FA, Lankisch TO, Winkler M, Klempnauer J et al (2013) Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy 45(6):433–438
Mennigen R, Harting C, Lindner K, Vowinkel T, Rijcken E, Palmes D et al (2015) Comparison of endoscopic vacuum therapy versus stent for anastomotic leak after esophagectomy. J Gastrointest Surg 19(7):1229–1235
Author information
Authors and Affiliations
Contributions
MB and SHC have written the manuscript. HF, TH, SB, JML, AHH, and WS have contributed largely to idea and proposal, evaluation and analysis of data. CJB has reviewed the manuscript critically.
Corresponding author
Ethics declarations
Disclosure
Marc Bludau, Hans F. Fuchs, Till Herbold, Felix Popp, Christiane J. Bruns, Arnulf H. Hölscher, Jessica M. Leers, Wolfgang Schröder, Hakan Alakus, Martin K. H. Maus, and Seung-Hun Chon have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (MP4 111340 KB)
Rights and permissions
About this article
Cite this article
Bludau, M., Fuchs, H.F., Herbold, T. et al. Results of endoscopic vacuum-assisted closure device for treatment of upper GI leaks. Surg Endosc 32, 1906–1914 (2018). https://doi.org/10.1007/s00464-017-5883-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5883-4