Abstract
Background and goals
Missed adenomas are likely to be located in the proximal colon and failure to detect these lesions might explain the occurrence of a certain percentage of interval carcinomas. Though studies have demonstrated increased detection of significant neoplastic lesions in colonoscopic examinations where the withdrawal time is 6 min or more, there are no recommendations on how much time to spend in each colonic segment. The aim of the trial was to find ways to reduce the number of lesions missed in the proximal segments of the colon assessing the difference in adenoma detection rate (ADR) between two colonoscopic withdrawal timed techniques.
Study
This was a randomized trial in a university hospital. Population was composed of patients referred for screening colonoscopy. The Main Outcome measurements was ADRs for patients subjected to a timed colonoscopy with specific withdrawal times, with special interest in the proximal colon, and implying a minimum of 2-min withdrawal delay in the cecum and right colon, a 1-min delay time in the transverse colon, and a minimum additional 3-min delay time in the left colon, as compared to a standard timed colonoscopy with free withdrawal delay time of at least 6 min.
Results
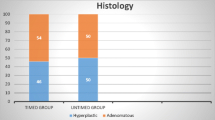
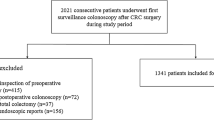
A total of 1160 patients were included. Eleven were initially excluded due to incomplete colonoscopies. Of the remaining 1149 patients, 573 were randomized to the group with fixed withdrawal times (Group A) and 576 to conventional withdrawal (Group B). Median age was 57 years (SD 6), a total of 634 (55.2%) were male patients and the mean withdrawal time was 7:05 min (SD 1 min). Seven hundred and eighty-one adenomas/serrated lesions were found in 470 patients (1.66 per patient), with 28 advanced lesions and 3 adenocarcinomas. Global ADR was 41% with no significant statistical differences between the two groups (42.1% vs 39.8%, p 0.43), respectively. A multivariate analysis showed clear relation between the finding of adenomas and higher BBPS ratings (Adjusted Odds Ratio [aOR] 0.92, p 0.05), age (aOR 1.03, p 0.01), male sex (aOR 1.51, p 0.001), and time of withdrawal (aOR 1.17, p 0.001), while no association was observed with either withdrawal technique (aOR 0.89, IC 95% 0.70–1.03, p 0.32). There was no statistical significant difference between the two groups concerning the finding of proximal lesions (cOR 0.93, CI 95% 0.71–1.20, p 0.56) (aOR 0.89, CI 95% 0.69–1.17, p 0.41) or serrated polyps (cOR 0.81, CI 95% 0.51–1.27, p 0.35) (aOR 0.81, IC 95% 0.51–1.28, p 0.36).
Conclusions
Fixed withdrawal times did not prove to lead to an increase in the number of detected adenomas. Nevertheless, our study supports previous reports stating that longer withdrawal times are indeed associated with better proximal and distal adenoma detection.


Similar content being viewed by others
References
Zauber AG, Winawer SJ, O’Brien MJ et al (2012) Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 366(8):687–696
Winawer SJ, Zauber AG, Nan Ho M et al (1993) Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med 329(27):1977–1981
Thiis-Evensen E, Hoff GS, Sauar J et al (1999) Population-based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Telemark Polyp Study I. Scand J Gastroenterol 34(4):414–420
Manser CN, Bachmann LM, Brunner J et al (2012) Colonoscopy screening markedly reduces the occurrence of colon carcinomas and carcinoma- related death: a closed cohort study. Gastrointest Endosc 76(1):110–117
Bressler B, Paszat LF, Chen Z et al (2007) Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology 132(1):96–102
Kaminski MF, Regula R, Kraszewski E et al (2010) Quality Indicators for colonoscopy and the risk of interval cancer. N Engl J Med 362:1795–1803
Pabby A, Schoen RE, Weissfeld JL et al (2005) Analysis of colorectal cancer occurrence during surveillance colonoscopy in the dietary Polyp Prevention Trial. Gastrointest Endosc 61(3):385–391
Rex DK, Bond JH, Winawer S et al (2002) Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 97(6):1296–1308
Rex DK, Petrini JL, Baron TH et al (2006) Quality indicators for colonoscopy. Gastrointest Endosc 63(4):s16–s28
Barclay RL, Vicari JJ, Doughty AS et al (2006) Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 355:2533–2541
Rex DK (2000) Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointest Endosc 51(1):33–36
Lai EJ, Calderwood AH, Doros G et al (2009) The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 69(3):620–625
Calderwood AH, Jacobson BC (2010) Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc 72(4):686–692
de Wijkerslooth TR, Stoop EM, Bossuyt PM et al (2013) Differences in proximal serrated polyp detection among endoscopists are associated with variability in withdrawal time. Gastrointest Endosc 77(4):617–623
Sanchez W, Harewood GC, Petersen BT (2004) Evaluation of polyp detection in relation to procedure time of screening or surveillance colonoscopy. Am J Gastroenterol 99(10):1941–1945
Jover R, Zapater P, Polanía E et al (2013) Modifiable endoscopic factors that influence the adenoma detection rate in colorectal cancer screening colonoscopies. Gastrointest Endosc 77(381–389):e1
Nishihara R, Wu K, Lochhead P et al (2013) Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 369(12):1095–1105
Lakoff J, Paszat LF, Saskin R et al (2008) Risk of Developing proximal versus distal colorectal cancer after a negative colonoscopy: a population-based study. Clin Gastroenterol Hepatol 6(10):1117–1121
Jacob BJ, Moineddin R, Sutradhar R et al (2012) Effect of colonoscopy on colorectal cancer incidence and mortality: an instrumental variable analysis. YMGE 76(2):355–364.e1
Singh H, Nugent Z, Demers AA et al (2010) The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. YGAST 139(4):1128–1137
Singh H, Nugent Z, Mahmud SM et al (2009) Predictors of colorectal cancer after negative colonoscopy: a population-based study. Am J Gastroenterol 105(3):663–673
Laiyemo AO, Doubeni C, Sanderson AK II et al (2011) Likelihood of missed and recurrent adenomas in the proximal versus the distal colon. Gastrointest Endosc 74(2):253–261
Robertson DJ, Greenberg ER, Beach M et al (2005) Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology 129(1):34–41
Farrar WD, Sawhney MS, Nelson DB et al (2006) Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol 4(10):1259–1264
Brenner H, Chang-Claude J, Seiler CM et al (2012) Interval cancers after negative colonoscopy: population-based case-control study. Gut 61(11):1576–1582
Ahn SB, Han DS, Bae JH et al (2012) The miss rate for colorectal adenoma determined by quality-adjusted, back-to-back colonoscopies. Gut Liver 6(1):64–70
Rex DK, Cutler CS, Lemmel GT et al (1997) Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. YGAST 112(1):24–28
Rex DK, Petrini JL, Baron TH et al (2006) Quality indicators for colonoscopy. Am J Gastroenterol 101:873–885
Corley DA, Jensen CD, Marks AR et al (2014) Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 370(14):1298–1306
Froehlich F, Wietlisbach V, Gonvers J-J et al (2005) Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. YMGE 61:378–384
Harewood GC, Sharma VK, de Garmo P (2003) Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 58:76–79
Menees SB, Kim HM, Eliot EE et al (2013) The impact of fair colonoscopy preparation on colonoscopy use and adenoma miss rates in patients undergoing outpatient colonoscopy. Gastrointest Endosc 78(3):510.43–516.43
Hassan C, Bretthauer M, Kaminski M et al (2013) Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 45(02):142–155
Parra-Blanco A, Nicolas-Perez D, Gimeno-Garcia A et al (2006) The timing of bowel preparation before colonoscopy determines the quality of cleansing. World J Gastroenterol 12(38):6161–6166
Cohen LB (2010) Split dosing of bowel preparations for colonoscopy: an analysis of its efficacy, safety, and tolerability. Gastrointest Endosc 72(2):406–412
American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society, 2015. 2014;24;1–56
Boroff ES, Gurudu SR, Hentz JG et al (2013) Polyp and adenoma detection rates in the proximal and distal colon. Am J Gastroenterol 108(6):993–999
Lewis JD, Ng K, Hung KE et al (2003) Detection of proximal adenomatous polyps with screening sigmoidoscopy: a systematic review and meta-analysis of screening colonoscopy. Arch Intern Med 163(4):413–420
Tanaka Y, Yamano H, Yamamoto E et al (2017) Endoscopic and molecular characterization of colorectal sessile serrated adenoma/polyps with cytology dysplasia. Gastrointest Endosc 86(6):1131–1138
Kolb JM, Soetikno RM, Rao AK et al (2017) Detection, diagnosis, and resection of sessile serrated adenomas and polyps. Gastroenterology 153:646–648
Saito S, Tajiri H, Ikegami M (2015) Serrated polyps of the colon and rectum: endoscopic features including image enhance endoscopy. World J Gastrointest Endosc 7(9):860–871
East JE, Atkin WS, Bateman AC et al (2017) British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut 66:1181–1196
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Eduardo Coghlan, Luis Laferrere, Juan Manuel Marini, German Rainero, Maria Lourdes Posadas Martinez, Alberto San Roman, and Angel Nadales have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Coghlan, E., Laferrere, L., Zenon, E. et al. Timed screening colonoscopy: a randomized trial of two colonoscopic withdrawal techniques. Surg Endosc 34, 1200–1205 (2020). https://doi.org/10.1007/s00464-019-06873-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06873-0