Abstract
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a debilitating side effect resulting from neurotoxic chemotherapeutic agents. This study aimed to assess the efficacy and safety of an oral B group vitamin compared to placebo, in preventing the incidence of CIPN in cancer patients undergoing neurotoxic chemotherapy.
Methods
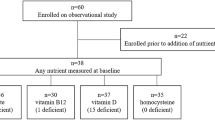
A pilot, randomised, placebo-controlled trial was conducted. Newly diagnosed cancer patients prescribed with taxanes, oxaliplatin or vincristine were invited to participate. A total of 71 participants (female 68 %, male 32 %) were enrolled into the study and randomised to the B group vitamin (n = 38) arm or placebo (n = 33). The data from 47 participants were eligible for analysis (B group vitamins n = 27, placebo n = 22). The primary outcome measure was the total neuropathy score assessed by an independent neurologist. Secondary outcome measures included serum vitamin B levels, quality of life, pain inventory and the patient neurotoxicity questionnaires. Outcome measures were conducted at baseline, 12, 24 and 36 weeks.
Results
The total neuropathy score (TNS) demonstrated that a B group vitamin did not significantly reduce the incidence of CIPN compared to placebo (p = 0.73). Statistical significance was achieved for patient perceived sensory peripheral neuropathy (12 weeks p = 0.03; 24 weeks p = 0.005; 36 weeks p = 0.021). The risk estimate for the Patient Neurotoxicity Questionnaire (PNQ) was also statistically significant (OR = 5.78, 95 % CI = 1.63–20.5). The European Organisation of Research and Treatment of Cancer (EORTC) quality of life, total pain score and pain interference showed no significance (p = 0.46, p = 0.9, p = 0.37 respectively). A trend was observed indicating that vitamin B12 may reduce the onset and severity of CIPN.
Conclusion
An oral B group vitamin as an adjunct to neurotoxic chemotherapy regimens was not superior to placebo (p > 0.05) for the prevention of CIPN. Patients taking the B group vitamin perceived a reduction in sensory peripheral neuropathy in the PNQ. Moreover, a robust clinical study is warranted given that vitamin B12 may show potential in reducing the onset and severity of CIPN.
Trial number: ACTRN12611000078954
Protocol number: UH2010000749


Similar content being viewed by others
References
Park SB, Goldstein D, Krishnan AV, et al. (2013) Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 63:419–437
Bhagra A, Rao RD (2007) Chemotherapy-induced neuropathy. Curr Oncol Rep 9:290–299
Cavaletti G, Nicolini G, Marmiroli P (2008) Neurotoxic effects of antineoplastic drugs: the lesson of pre-clinical studies. Front Biosci 13:3506–3524
Cavaletti G, Marmiroli P (2004) Chemotherapy-induced peripheral neurotoxicity. Expert Opin Drug Saf 3:535–546
Armstrong T, Almadrones L, Gilbert MR (2005) Chemotherapy-induced peripheral neuropathy. Oncol Nurs Forum 32:305–311
Cersosimo RJ (1989) Cisplatin neurotoxicity. Cancer Treat Rev 16:195–211
Roelofs RI, Hrushesky W, Rogin J, et al. (1984) Peripheral sensory neuropathy and cisplatin chemotherapy. Neurology 34:934–938
Cancer Treatment & Survivorship Facts & Figures 2014-2015. (2015) http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf
Scott J, Weir D (1994) Folate/vitamin B12 inter-relationships. Essays Biochem 28:63–72
Soloman L. (2016) Functional vitamin B12 deficiency in advanced malignancy: implications for the management of neuropathy and neuropathic pain. Support Care Cancer. March.
Vu T, Amin J, Ramos M, et al. (1993) New assay for the rapid determination of plasma holotranscobalamin II levels: preliminary evaluation in cancer patients. Am J Hematol 42:202–211
Schloss J, Colosimo M, Airey C, Vitetta L (2015) Chemotherapy-induced peripheral neuropathy (CIPN) and vitamin B12 deficiency. Support Care Cancer 23(7):1843–1850
Kim HI, Hyung WJ, Song KJ, Choi SH, Kim CB, Noh SH (2011) Oral vitamin B12 replacement: an effective treatment for vitamin B12 deficiency after total gastrectomy in gastric cancer patients. Ann Surg Oncol 18(14):3711–3717
Ghavanini AA, Kimpinski K (2014) Revisiting the evidence for neuropathy caused by pyrdioxine deficiency and excess. J Clin Neuromuscul Dis 16(1):25–31
Ang CD, Alviar MJ, Dans AL, Bautista-Velz GG, et al. (2008) Vitamin B for treating peripheral neuropathy. Cochrane Database Syst Rev 16(3):CD004573
Cavaletti G, Alberti P, Frigeni B (2011) Chemotherapy-induced neuropathy. Curr Treat Options Neurol 13:180–190
Smith EM, Beck SL, Cohen L (2008) The total neuropathy score: a tool for measuring chemotherapy-induced peripheral neuropathy. Oncol Nurs Forum 35:96–102
Cleeland CS. (1989) Measurement of pain by subjective report. In: Chapman CR, Loeser JD, editors. Issues in pain measurement. Advances in pain research and therapy, vol. 12. New York: Raven Press. P391–403
Groenvold M, Klee MC, Sprangers MAG, Aaronson NK (1997) Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J of Clin Epid 40(4):441–450
Shimozuma K, Ohashi Y, Takeuchi A, Aranishi T, Satoshi M, et al. (2009) Feasibility and validity of the patient neurotoxicity questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N-SAS BC 02. Support Care Cancer 17:1483
Argyriou AA, Chroni E, Koutras E, et al. (2005) Vitamin E for prophylaxis against chemotherapy-induced neuropathy: a randomized controlled trial. Neurology 64:26–31
Wang WS, Lin JK, Lin TC, et al. (2007) Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist 12:312–319
Pace A, Giannarelli D, Galie E, et al. (2010) Vitamin e neuroprotection for cisplatin neuropathy: a randomized, placebo-controlled trial. Neurology 74:762–766
Argyriou AA, Chroni E, Koutras A, et al. (2006) A randomized controlled trial evaluating the efficacy and safety of vitamin E supplementation for protection against cisplatin-induced peripheral neuropathy: final results. Support Care Cancer 14:1134–1140
Gamelin L, Boisdron-Celle M, Delva R, et al. (2004) Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5-fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res 10:4055–4061
Gedlicka C, Scheithauer W, Schull B, et al. (2002) Effective treatment of oxaliplatin-induced cumulative polyneuropathy with alpha-lipoic acid. J Clin Oncol 20:3359–3361
Milla P, Airoldi M, Weber G, et al. (2009) Administration of reduced glutathione in FOLFOX4 adjuvant treatment for colorectal cancer: effect on oxaliplatin pharmacokinetics, Pt-DNA adduct formation, and neurotoxicity. Anti-Cancer Drugs 20:396–402
Nordstokke DW, Zumbo B, Cairns SL, et al. (2011) The operating characteristics of the nonparametric Levene test for equal variances with assessment and evaluation data. Practical Assessment Research Evaluation. 16:ISSN 1531–7714.
Nordstokke DW, Zumbo B (2010) A new nonparametric Levene test for equal variances. Psicológica 31:401–430
Schloss JM, Colosimo M, Airey C, Masci PP, Linnane AW, Vitetta L (2013) Nutraceuticals and chemotherapy induced peripheral neuropathy (CIPN): a systematic review. Clin Nutr 32(6):888–893 Erratum in: Clin Nutr. 2015 Feb:34(1):167
Herbert V. (1996) present knowledge in nutrition. 7th Edition ed. Vitamin B12, ed F.L. Ziegler EE. Washington, DC, USA. International Life Sciences Institute Press
Kayl AE, Meyers CA (2006) Side-effects of chemotherapy and quality of life in ovarian and breast cancer patients. Curr Opin Obstet Gynecol 18(1):24–28
Acknowledgments
We would like to thank Bio Concepts Pty Ltd. for supplying the supplemental product and assisting the research position to conduct this trial. Acknowledgement also goes to all the oncology and neurological staff at the Princess Alexandra Hospital and the members of the former Centre for Integrative and Clinical Medicine at the University of Queensland for their assistance during the study. A special acknowledgement goes to Dr. Samantha Coulson for her detailed editing of the manuscript and assistance with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
The study design complied with the Helsinki Declaration and was approved by the University of Queensland’s Human Research Ethics Committee (UH 2010000749) and by the Princess Alexandra Hospital’s Human Research Ethics Committee (HREC/10/QPAH/140).
Funding
The authors have received no financial support for the research, authorship and/or publication of this article.
Conflicts of interest
Luis Vitetta has received National Institute of Complementary Medicine and National Health and Medical Research Council of Australia competitive funding and industry support for research into nutraceuticals and herbal medicines. The authors declare no other potential conflicts of interest with respect to research, authorship and/or publication of this article.
Rights and permissions
About this article
Cite this article
Schloss, J.M., Colosimo, M., Airey, C. et al. A randomised, placebo-controlled trial assessing the efficacy of an oral B group vitamin in preventing the development of chemotherapy-induced peripheral neuropathy (CIPN). Support Care Cancer 25, 195–204 (2017). https://doi.org/10.1007/s00520-016-3404-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3404-y