Abstract
Background
Nonalcoholic fatty liver disease ranges from simple steatosis to nonalcoholic steatohepatitis (NASH). Kupffer cells play a central role in promoting hepatic inflammation, which leads to the development of NASH. We investigated the anti-inflammatory effect of hepatic vagus-mediated stimulation of the α7 nicotinic acetylcholine receptor (α7nAChR) on Kupffer cells in NASH pathogenesis.
Methods
Wild-type (WT) mice undergoing hepatic vagotomy (HV) were fed a methionine- and choline-deficient (MCD) diet for 1 week. α7nAChR knockout (α7KO) chimeric mice were generated by transplanting α7KO bone marrow cells into irradiated and Kupffer cell-deleted WT recipients. Kupffer cells were isolated from WT mice and treated with α7nAChR agonist under stimulation by lipopolysaccharide and/or palmitic acid.
Results
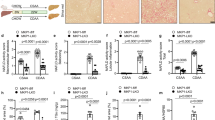
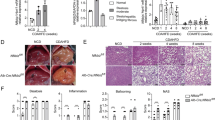
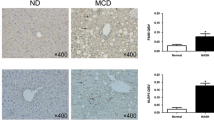
HV aggravated MCD diet-induced NASH in both steatosis and inflammation. The hepatic inflammatory response, including the upregulation of tumor necrosis factor alpha (TNFα), interleukin (IL)-12, and monocyte chemoattractant protein 1 (MCP-1), was accelerated in HV mice, accompanied by the downregulation of PPARα pathway genes. Kupffer cells were highly activated via the phosphorylation and nuclear translocation of nuclear factor-kappa B (NF-κB) in MCD diet-fed HV mice. The α7nAchR agonist suppressed the inflammatory response of primary Kupffer cells induced by lipopolysaccharide and palmitic acid by attenuating the NF-κB cascade. α7KO chimeric mice fed an MCD diet for 1 week developed advanced NASH with highly activated Kupffer cells. The hepatic expression of TNFα, IL-12, and MCP-1 was upregulated in α7KO chimeric mice, accompanied by abnormal lipid metabolism.
Conclusions
Hepatic vagus activity regulates the inflammatory response of Kupffer cells via α7nAChR in NASH development.






Similar content being viewed by others
Abbreviations
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- TLR:
-
Toll-like receptor
- IL:
-
Interleukin
- TNFα:
-
Tumor necrosis factor alpha
- MCP-1:
-
Monocyte chemoattractant protein 1
- α7nAChR:
-
α7 nicotinic acetylcholine receptor
- STAT3:
-
Signal transducer and activator of transcription 3
- NF-κB:
-
Nuclear factor-kappa B
- MCD:
-
Methionine- and choline-deficient
- WT:
-
Wild type
- α7KO:
-
α7nAChR knockout
- HV:
-
Hepatic vagotomy
- CT:
-
Control
- BM:
-
Bone marrow
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- LPS:
-
Lipopolysaccharide
- PPARα:
-
Peroxisome proliferator-activated receptor alpha
- AOX:
-
Acyl-CoA oxidase
- L-FABP:
-
Liver-type fatty acid binding protein
- SREBF-1c:
-
Sterol regulatory element binding factor 1c
- FAS:
-
Fatty acid synthase
- G6pc:
-
Glucose 6-phosphatase
- PEPCK:
-
Phosphoenolpyruvate carboxykinase 1
- GFP:
-
Green fluorescent protein
References
Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–89.
Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143(10):722–8.
Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–23.
Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51(5):1820–32.
Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–9.
Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–5.
Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–46.
Li Z, Yang S, Lin H, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37(2):343–50.
Lin HZ, Yang SQ, Chuckaree C, et al. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6(9):998–1003.
Maher JJ, Leon P, Ryan JC. Beyond insulin resistance: innate immunity in nonalcoholic steatohepatitis. Hepatology. 2008;48(2):670–8.
Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48(1):322–35.
Miura K, Yang L, van Rooijen N, et al. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57(2):577–89.
Rivera CA, Adegboyega P, van Rooijen N, et al. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47(4):571–9.
Miura K, Kodama Y, Inokuchi S, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139(1):323–34.
Leroux A, Ferrere G, Godie V, et al. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J Hepatol. 2012;57(1):141–9.
Huang W, Metlakunta A, Dedousis N, et al. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59(2):347–57.
Kremer M, Thomas E, Milton RJ, et al. Kupffer cell and interleukin-12-dependent loss of natural killer T cells in hepatosteatosis. Hepatology. 2010;51(1):130–41.
Tosello-Trampont AC, Landes SG, Nguyen V, et al. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J Biol Chem. 2012;287(48):40161–72.
Mandrekar P, Ambade A, Lim A, et al. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54(6):2185–97.
Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–62.
Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–9.
Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9(6):418–28.
Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–8.
de Jonge WJ, van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6(8):844–51.
Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14(9–10):567–74.
Guarini S, Altavilla D, Cainazzo MM, et al. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107(8):1189–94.
Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265(6):663–79.
Wang X, Yang Z, Xue B, et al. Activation of the cholinergic antiinflammatory pathway ameliorates obesity-induced inflammation and insulin resistance. Endocrinology. 2011;152(3):836–46.
Satapathy SK, Ochani M, Dancho M, et al. Galantamine alleviates inflammation and other obesity-associated complications in high-fat diet-fed mice. Mol Med. 2011;17(7–8):599–606.
Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex—linking immunity and metabolism. Nat Rev Endocrinol. 2012;8(12):743–54.
Lam TK, Pocai A, Gutierrez-Juarez R, et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11(3):320–7.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5.
Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21.
Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509.
Tamaki N, Hatano E, Taura K, et al. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol. 2008;294(2):G498–505.
Yi CX, la Fleur SE, Fliers E, et al. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta. 2010;1802(4):416–31.
Uno K, Katagiri H, Yamada T, et al. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science. 2006;312(5780):1656–9.
Pocai A, Obici S, Schwartz GJ, et al. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005;1(1):53–61.
Kimura K, Tanida M, Nagata N, et al. Central insulin action activates Kupffer cells by suppressing hepatic vagal activation via the nicotinic alpha 7 acetylcholine receptor. Cell Rep. 2016;14(10):2362–74.
Zhou Z, Liu YC, Chen XM, et al. Treatment of experimental non-alcoholic steatohepatitis by targeting α7 nicotinic acetylcholine receptor-mediated inflammatory responses in mice. Mol Med Rep. 2015;12(5):6925–31.
Fernández-Alvarez A, Alvarez MS, Gonzalez R, et al. Human SREBP1c expression in liver is directly regulated by peroxisome proliferator-activated receptor alpha (PPARalpha). J Biol Chem. 2011;286(24):21466–77.
Yoshikawa T, Ide T, Shimano H, et al. Cross-talk between peroxisome proliferator-activated receptor (PPAR) alpha and liver X receptor (LXR) in nutritional regulation of fatty acid metabolism. I. PPARs suppress sterol regulatory element binding protein-1c promoter through inhibition of LXR signaling. Mol Endocrinol. 2003;17(7):1240–54.
Pal D, Dasgupta S, Kundu R, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18(8):1279–85.
Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28(4):360–9.
Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3(4):445–51.
Rosas-Ballina M, Olofsson PS, Ochani M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334(6052):98–101.
Rinella ME, Elias MS, Smolak RR, et al. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008;49(5):1068–76.
Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8(1):35–44.
Guerrerio AL, Colvin RM, Schwartz AK, et al. Choline intake in a large cohort of patients with nonalcoholic fatty liver disease. Am J Clin Nutr. 2012;95(4):892–900.
Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol. 2012;28(2):159–65.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 15K15495), a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 26461909), and a grant from the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nishio, T., Taura, K., Iwaisako, K. et al. Hepatic vagus nerve regulates Kupffer cell activation via α7 nicotinic acetylcholine receptor in nonalcoholic steatohepatitis. J Gastroenterol 52, 965–976 (2017). https://doi.org/10.1007/s00535-016-1304-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-016-1304-z