Abstract
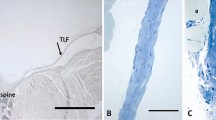
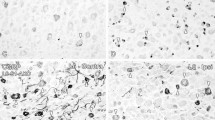
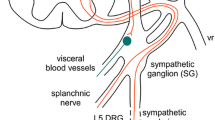
A considerable number of patients complain about pain after lumbar surgery. The spinal dura mater has been debated as a possible source of this pain. However, there is no information if laminectomy influences the nociceptive sensory innervation of the dura. Therefore, we quantitatively evaluated the density of SP- and CGRP-immunopositive nerve fibers in the dura mater lumbalis in an animal model of laminectomy. Twelve adult Lewis rats underwent laminectomy, in six of them the exposed dura was covered by an autologous fat graft. Further six animals without surgical treatment served as controls. Six weeks after surgery, the animals were perfused and the lumbar dura was processed immunohistochemically for the detection of CGRP- and SP-containing nerve fibers. In controls, the peptidergic nerve fibers were found predominantly in the ventral but rarely in the dorsal dura mater lumbalis. After laminectomy, the density of SP- and CGRP-immunopositive neurons significantly increased in ventral as well as in dorsal parts of the dura. Axonal spines could be observed in some cases at the site of laminectomy. The application of autologous fat grafts failed to inhibit the significant increase in the density of peptidergic afferents. Thus, we have provided the first evidence that laminectomies induce an increase in the density of putative nociceptive SP- and CGRP-immunopositive neurons in the lumbar dura mater ascribable to an axonal sprouting of fine nerve fibers. This effect was not prevented by using autologous fat grafts. It is conceivable that the neuronal outgrowth of nociceptive afferents is a cause of low back pain observed after lumbar surgery.





Similar content being viewed by others
References
Ahmed M, Bjurholm A, Kreicbergs A, Schultzberg M (1991) SP- and CGRP-immunoreactive nerve fibers in the rat lumbar spine. Neuroorthopedics 12:19–28
Ahmed M, Bjurholm A, Kreicbergs A, Schultzberg M, Neuropeptide Y (1993) Tyrosine hydroxylase and vasoactive intestinal polypeptide-immunoreactive nerve fibers in the vertebral bodies, discs, dura mater, and spinal ligaments of the rat lumbar spine. Spine 18(2):268–273. doi:10.1097/00007632-199302000-00016
Albers KM, Wright DE, Davis BM (1994) Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J Neurosci 14:1422–1432
Benner B, Ehni G (1978) Spinal arachnoiditis. Spine 3:40–44. doi:10.1097/00007632-197803000-00009
Bernsmann K, Krämer J, Ziozios I, Wehmeier J, Wiese M (2001) Lumbar micro disc surgery with and without autologous fat graft. A prospective randomized trial evaluated with reference to clinical and social factors. Arch Orthop Trauma Surg 121(8):476–480. doi:10.1007/s004020100277
Blikra G (1969) Intradural herniated lumbar disc. J Neurosurg 31(6):676–679
Bogduk N (1992) The sources of low back pain. In: Jayson IV (ed) The lumbar spine and back pain. Churchill Livingston, Edinburgh, pp 61–88
Burton CV (1978) Lumbosacral arachnoiditis. Spine 3:24–30. doi:10.1097/00007632-197803000-00006
Carmeliet P, Tessier-Lavigne M (2005) Common mechanisms of nerve and blood vessel wiring. Nature 436(7048):193–200. doi:10.1038/nature03875
Ceviz A, Arslan A, Ak HE, Inalöz S (1997) The effect of urokinase in preventing the formation of epidural fibrosis and/or leptomeningeal arachnoiditis. Surg Neurol 47(2):124–127. doi:10.1016/S0090-3019(96)00038-9
Cyriax J (1978) Dural pain. Lancet 1(8070):919–921. doi:10.1016/S0140-6736(78)90693-1
Davis BM, Fundin BT, Albers KM, Goodness TP, Cronk KM, Rice FL (1997) Overexpression of nerve growth factor in skin causes preferential increases among innervation to specific sensory targets. J Comp Neurol 387:489–506. doi:10.1002/(SICI)1096-9861(19971103)387:4<489::AID-CNE2>3.0.CO;2-Z
Diamond J, Foerster A, Holmes M, Coughlin M (1992) Sensory nerves in adult rats regenerate and restore sensory function to the skin independently of endogenous NGF. J Neurosci 12:1467–1476
Diamond J, Holmes M, Coughlin M (1992) Endogenous NGF and nerve impulses regulate the collateral sprouting of sensory axons in the skin of the adult rat. J Neurosci 12:1454–1466
Dickson A, Avelino A, Cruz F, Ribeiro-da-Silva A (2006) Peptideregic sensory and parasympathetic fiber sprouting in the mucosa of the rat urinary bladder in a chronic model of cyclophosphamide-induced cystitis. Neuroscience 139:671–685. doi:10.1016/j.neuroscience.2005.11.050
DiFazio FA, Nichols JB, Pope MH, Frymoyer JW (1995) The use of expanded polytetrafluoroethylene as an interpositional membrane after lumbar laminectomy. Spine 20(9):986–991. doi:10.1097/00007632-199505000-00002
Edgar MA, Nundy S (1966) Innervation of the spinal dura mater. J Neurol Neurosurg Psychiatry 29:530–534
El-Mahdi MA, Abdel Latif FY, Janko M (1981) The spinal nerve root “innervation”, and a new concept of the clinicopathological interrelations in back pain and sciatica. Neurochirurgia (Stuttg) 24(4):137–141
Federoff HJ, Grabczyk E, Fishman MC (1988) Dual regulation of GAP-43 gene expression by nerve growth factor and glucocorticoids. J Biol Chem 263(36):19290–19295
Gambardella G, Gervasio O, Zaccone C, Puglisi E (2005) Prevention of recurrent radicular pain after lumbar disc surgery: a prospective study. Acta Neurochir Suppl (Wien) 92:151–154
Geis A, Rohleder N, Kirschbaum C, Steinbach K, Bauer HW, Anton F (2005) Predicting the failure of disc surgery by a hypofunctional HPA axis: evidence from a prospective study on patients undergoing disc surgery. Pain 114:104–117. doi:10.1016/j.pain.2004.12.007
Gloster A, Diamond J (1992) Sympathetic nerves in adult rats regenerate normally and restore pilomotor function during an anti-NGF treatment that prevents their collateral sprouting. J Comp Neurol 326:363–374. doi:10.1002/cne.903260305
Gloster A, Diamond J (1995) NGF-dependent and NGF-independent recovery of sympathetic function after chemical sympathectomy with 6-hydroxydopamine. J Comp Neurol 359:586–594. doi:10.1002/cne.903590406
Gorgulu A, Simsek O, Cobanoglu S, Imer M, Parsak T (2004) The effect of epidural free fat graft on the outcome of lumbar disc surgery. Neurosurg Rev 27:181–184. doi:10.1007/s10143-003-0310-9
Grelik C, Bennett GJ, Ribeiro-da-Silva A (2005) Autonomic fibre sprouting and changes in nociceptive sensory innervation in the rat lower lip skin following chronic constriction injury. Eur J Neurosci 21(9):2475–2487. doi:10.1111/j.1460-9568.2005.04089.x
Groen GJ, Baljet B, Drukker J (1988) The innervation of the spinal dura mater: anatomy and clinical implications. Acta Neurochir (Wien) 92(1–4):39–46. doi:10.1007/BF01401971
Harris LW, Purves D (1989) Rapid remodeling of sensory endings in the corneas of living mice. J Neurosci 9(6):2210–2214
Hoyle GW, Graham RM, Finkelstein JB, Nguyen KP, Gozal D, Friedman M (1998) Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol 18(2):149–157
Jensen TT, Asmussen K, Berg-Hansen EM, Laurutzen B, Manniche C, Vinterberg H et al (1996) First-time operation for lumbar disc herniation with or without free fat transplantation Prospective triple-blind randomized study with reference to clinical factors and enhanced computed tomographic scan 1 year after operation. Spine 21(9):1072–1076. doi:10.1097/00007632-199605010-00016
Kallakuri S, Cavanaugh J, Blagoev D (1998) An immunohistochemical study of innervation of lumbar spinal dorsal dura and longitudinal ligaments. Spine 23(4):403–411. doi:10.1097/00007632-199802150-00001
Kawamoto K, Matsuda H (2004) Nerve growth factor and wound healing. Prog Brain Res 146:369–384
Keller JT, Marfurt CF (1991) Peptidergic and serotoninergic innervations of the rat dura mater. J Comp Neurol 309:515–534. doi:10.1002/cne.903090408
Kinkelin I, Mötzing S, Koltzenburg M, Bröcker E-B (2000) Increase in NGF content and nerve fiber sprouting in human allergic contact eczema. Cell Tissue Res 302:31–37. doi:10.1007/s004410000202
Kiviluoto O (1976) Use of free fat transplants to prevent epidural scar formation: an experimentsl study. Acta Orthop Scand Suppl 164:73–75
Konnai Y (1994) Nerve innervation of peptidergic fibers in the spinal dura mater of rats. Acta Anat Nipponica 69:89
Konnai Y, Honda T, Sekiguchi Y, Kikuchi S, Sugiura Y (2000) Sensory innervation of the lumbar dura mater passing through the sympathetic trunk in rats. Spine 25(7):776–782. doi:10.1097/00007632-200004010-00004
Kosten TA, Ambrosio E (2002) HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology 27(1–2):35–69. doi:10.1016/S0306-4530(01)00035-X
Kuivila TE, Berry JL, Bell GR, Steffee AD (1988) Heparinized materials for control of the formation of the laminectomy membrane in experimental laminectomies in dogs. Clin Orthop Relat Res 236:166–173
Kumar R, Berger R, Dunsker S, Keller J (1996) Innervation of the spinal dura: myth or reality? Spine 21(1):18–25. doi:10.1097/00007632-199601010-00004
Langenskiöld A, Kiviluoto O (1976) Prevention of epidural scar formation after operations on the lumbar spine by means of free fat transplants. A preliminary report. Clin Orthop Relat Res 115:92–95
Leslie TA, Emson PC, Dowd PM, Woolf CJ (1995) Nerve growth factor contributes to the up-regulation of growth-associated protein 43 and preprotachykinin. A messenger RNAs in primary sensory neurons following peripheral inflammation. Neuroscience 67:753–761. doi:10.1016/0306-4522(95)00101-N
Lindholm D, Hengerer B, Heumann R, Carroll P, Thoenen H (1990) Glucocorticoid hormones negatively regulate nerve growth factor expression in vivo and in cultured rat fibroblasts. Eur J Neurosci 2:795–801. doi:10.1111/j.1460-9568.1990.tb00471.x
Loeser J (1985) Pain due to nerve injury. Spine 10:232–235. doi:10.1097/00007632-198504000-00007
Matsuda H, Koyama H, Sato H, Sawada J, Itakura A, Tanaka A et al (1998) Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J Exp Med 187(3):297–306. doi:10.1084/jem.187.3.297
McCarron RF, Wimpee MW, Hudkins PG, Laros GS (1987) The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain. Spine 12(8):760–764. doi:10.1097/00007632-198710000-00009
Mearow KM, Kril Y (1995) Anti-NGF treatment blocks the upregulation of NGF receptor mRNA expression associated with collateral sprouting of rat dorsal root ganglion neurons. Neurosci Lett 184:55–58. doi:10.1016/0304-3940(94)11167-H
Meßlinger K, Hanesch U, Baumgärtel M, Trost B, Schmidt RF (1993) Innervation of the dura mater encephali of cat and rat: ultrastructure and CGRP/SP-like immunoreactivity. Anat Embryol (Berl) 188:219–237
Mosconi T, Krüger L (1996) Fixed-diameter polyethylene cuffs applied to the rat sciatic nerve induce a painful neuropathy: ultrastructural morphometric analysis of axonal alterations. Pain 64(1):37–57. doi:10.1016/0304-3959(95)00077-1
Oaklander AL, North RB (2001) Failed back surgery syndrome. In: Loeser JD (ed) Bonica`s management of pain. Williams & Wilkins, Philadelphia, pp 1540–1549
O’Connor TP, van der Kooy D (1988) Enrichment of a vasoactive neuropeptide (calcitonin gene related peptide) in the trigeminal sensory projection to the intracranial arteries. J Neurosci 8(7):2468–2476
Parke WW, Watanabe R (1990) Adhesions of the ventral lumbar dura. An adjunct source of discogenic pain? Spine 15(4):300–303. doi:10.1097/00007632-199004000-00010
Pedersen HE, Blunck CF, Gardner E (1956) The anatomy of lumbosacral posterior rami and meningeal branches of spinal nerve (sinu-vertebral nerves); with an experimental study of their functions. J Bone Joint Surg Am 38:377–391
Petrie JL, Ross JS (1996) Use of ADCON-L to inhibit postoperative fibrosis and related symptoms following lumbar disc surgery: a preliminary report. Eur Spine J 5(Suppl1):10–17. doi:10.1007/BF00298567
Ramer MS, Bisby MA (1999) Adrenergic innervation of rat sensory ganglia following proximal or distal painful sciatic neuropathy: distinct mechanisms revealed by anti-NGF treatment. Eur J Neurosci 11(3):837–846. doi:10.1046/j.1460-9568.1999.00491.x
Reinert A, Kaske A, Mense S (1998) Inflammation-induced increase in the density of neuropeptide-immunoreactive nerve endings in rat skeletal muscle. Exp Brain Res 121:174–180. doi:10.1007/s002210050449
Rossi F, van der Want JJ, Wiklund L, Strata P (1991) Reinnervation of cerebellar Purkinje cells by climbing fibres surviving a subtotal lesion of the inferior olive in the adult rat. II. Synaptic organization on reinnervated Purkinje cells. J Comp Neurol 308(4):536–554. doi:10.1002/cne.903080404
Saxler G, Krämer J, Barden B, Kurt A, Pförtner J, Bernsmann K (2005) The long-term clinical sequelae of incidental durotomy in lumbar disc surgery. Spine 30(20):2298–2302. doi:10.1097/01.brs.0000182131.44670.f7
Schofferman J, Reynolds J, Herzog R, Covington E, Dreyfuss P, O`Neill C (2003) Failed back surgery: etiology and diagnostic evaluation. Spine J 3(5):400–403. doi:10.1016/S1529-9430(03)00122-0
Sekiguchi Y, Kinnai Y, Kikuchi S, Sugiura Y (1996) An anatomic study of neuropeptide immunoreactivities in the lumbar dura mater after lumbar sympathectomy. Spine 21(8):925–930. doi:10.1097/00007632-199604150-00004
Songer MN, Rauschnig W, Carson EW, Pandit SM (1995) Analysis of peridural scar formation and its prevention after lumbar laminotomy and discectomy in dogs. Spine 20(5):571–580. doi:10.1097/00007632-199503010-00012
Stelzner DJ, Baisden RH, Goodman DC (1976) The ventral lateral geniculate nucleus, pars lateralis of the rat. Synaptic organization and conditions for axonal sprouting. Cell Tissue Res 170(4):435–454. doi:10.1007/BF00361703
Sternberg EM, Hill JM, Chrousos GP, Kamilaris T, Listwak SJ, Gold PW et al (1989) Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci USA 86:2374–2378. doi:10.1073/pnas.86.7.2374
Stilwell DL (1956) The nerve supply of the vertebral column and its associated structures in the monkey. Anat Rec 125(2):139–169. doi:10.1002/ar.1091250202
Vaquero J, Arias A, Oy S, Martinez R, Zurita M (1993) Effect of fibrin glue on postlaminectomy scar formation. Acta Neurochir (Wien) 120(3–4):159–163. doi:10.1007/BF02112036
Wiberg G (1949) Back pain in relation to the nerve supply of the intervertebral disc. Acta Orthop Scand 19:211–221
Wiklund L, Rossi F, Strata P, van der Want JJ (1990) The rat olivocerebellar system visualized in detail with anterograde PHA-L tracing technique, and sprouting of climbing fibers demonstrated after subtotal olivary lesions. Eur J Morphol 28(2–4):256–267
Wilkinson HA (1992) The Failed back surgery syndrome. Springer, New York, pp 1–2
Yen LD, Bennett GJ, Ribeiro-da-Silva A (2006) Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury. J Comp Neurol 495(6):679–690. doi:10.1002/cne.20899
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saxler, G., Brankamp, J., von Knoch, M. et al. The density of nociceptive SP- and CGRP-immunopositive nerve fibers in the dura mater lumbalis of rats is enhanced after laminectomy, even after application of autologous fat grafts. Eur Spine J 17, 1362–1372 (2008). https://doi.org/10.1007/s00586-008-0741-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-008-0741-7