Abstract
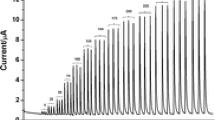
An amperometric detector with a gold nanoparticle modified carbon paste electrode (GNMCPE) was applied applied to flow injection analysis for the determination of acetaminophen. An obvious shift of the peak potential and increase of the current peak were observed for the GNMCPE in comparison to that of the bare carbon paste electrode. The experimental conditions, such as species of buffer, pH, flow rate, detection volume, injection volume, and injection time were investigated. Under the optimized conditions, the calibration curve was obtained over the concentration range of 0.1–80 mg L−1 of acetaminophen with a linear correlation coefficient of 0.9994. The detection limit (3σ) was estimated to be 0.05 mg L−1 (n = 7). The recoveries of acetaminophen were between 98.40% and 104.1%, and the relative standard deviation varied between 1.66% and 2.74% for the different samples. This method was applied to analyze six types of tablets obtained from a local drugstore. The contents of acetaminophen were found to be 0.498, 0.323, 0.249, 0.324, 0.319 and 0.323 g of each tablet, respectively. These results are consistent with the values obtained by high performance liquid chromatography.






Similar content being viewed by others
References
Adams RN (1958) Carbon paste electrodes. Anal Chem 30:1576
Kuwana T, French WH (1964) Electrooxidation or reduction of organic compounds into aqueous solutions using carbon paste electrode. Anal Chem 36:241
Wring SA, Hart JP, Birch BP (1990) Voltammetric behaviour of ascorbic acid at a graphite-epoxy composite electrode chemically modified with cobalt phthalocyanine and its amperometric determination in multivitamin preparations. Anal Chim Acta 229:63
Ulakhovich NA, Medyantseva EP, Budnikov GK (1993) Carbon paste electrode as a sensor in voltammetric analysis. J Anal Chem 48:682
Kalcher K, Kauffmann JM, Wang J, Svancara I, Vgtrass K, Neuhold C, Yang Z (1995) Sensors based on carbon-paste in electrochemical analysis—a review with particular emphasis on the period 1990–1993. Electroanal 7:5
Langer K, Barczyński P, Baksalary K, Filipiak M, Golczak S, Langer JJ (2007) A fast and sensitive continuous flow nanobiodetector based on polyaniline nanofibrils. Microchimica Acta 159:201
Wildgoose GG, Banks CE, Leventis HC, Compton RG (2006) Chemically modified carbon nanotubes for use in electroanalysis. Microchimica Acta 152:187
Zhao J, Yu JJ, Wang F, Hu SS (2007) Fabrication of gold nanoparticle-dihexadecyl hydrogen phosphate film on a glassy carbon electrode, and its application to glucose sensing. Microchimica Acta 156:277
Zhuang ZJ, Su XD, Yuan HY, Sun Q, Xiao D, Choi MMF (2008) An improved sensitivity non-enzymatic glucose sensor based on a CuO nanowire modified Cu electrode. The Analyst 133:126
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293
Liu Y, Geng T, Gao J (2007) Layer-by-layer immobilization of horseradish peroxidase on a gold electrode modified with colloidal gold nanoparticles. Microchimica Acta 161:241–248
Ding XQ, Yang M, Hu JB, Li QL, Mcdougall A (2007) Study of the adsorption of cytochrome c on a gold nanoparticle-modified gold electrode by using cyclic voltammetry, electrochemical impedance spectroscopy and chronopotentiometry. Microchimica Acta 158:65
Quintino Maria SM, Araki K, Toma HE, Angnes L (2002) Batch injection analysis utilizing modified electrodes with tetraruthenated porphyrin films for acetaminophen quantification. Electroanalysis 14:1629
Srivastava MK, Ahmed S, Singh D, Shukla IC (1985) Titrimetric determination of dipyrone and paracetamol with potassium hexacyanoferrate (III) in an acidic medium. Analyst 110:735
Bargot G, Auffret F, Burgot JL (1997) Determination of acetaminophen by thermometric titrimetry. Anal Chim Acta 343:125
Calatayud JM, Benito CG (1990) Flow-injection spectrofluorimetric determination of paracetamol. Anal Chim Acta 231:259
Vilchez JL, Blanc R, Avidad R, Navalón A (1995) Spectrofluorimetric determination of paracetamol in pharmaceuticals and biological fluids. J Pharm Biomed Anal 13:1119
Milch G, Szabó E (1991) Derivative spectrophotometric assay of acetaminophen and spectrofluorimetric determination of its main impurity. J Pharm Biomed Anal 9(10–12):1107
Ayaora Cañada MJ, Pascual Reguera MI, Ruiz Medina A, Fernández-de Córdova ML, Molina Díaz A (2000) Fast determination of paracetamol by using a very simple photometric flow-through sensing device. J Pharm Biomed Anal 22:59
Ni Y, Liu C, Kokot S (2000) Simultaneous kinetic spectrophotometric determination of acetaminophen and phenobarbital by artificial neural networks and partial least squares. Anal Chim Acta 419:185
Nagaraja P, Murthy KCS, Rangappa KS (1998) Spectrophotometric method for the determination of paracetamol and phenacetin. J Pharm Biomed Anal 17:501
Afshari JT, Liu TZ (2001) Rapid spectrophotometric method for the quantitation of acetaminophen in serum. Anal Chim Acta 443:165
Hewala II (1994) High-performance liquid chromatographic and derivative difference spectrophotometric methods for the determination of acetaminophen and its degradation product in aged pharmaceutical formulations. Anal Lett 27:561
Pérez JL, Bello MA (1999) Determination of paracetamol in dosage forms by non-suppressed ion chromatography. Talanta 48:1199
Dimitrova B, Doytchinova I, Zlatkova M (2000) Reversed-phase high-performance liquid chromatography for evaluating the distribution of pharmaceutical substances in suppository base-phosphate buffer system. J Pharm Biomed Anal 23:955
Ahrer W, Scherwenk E, Buchberger W (2001) Determination of drug residues in water by the combination of liquid chromatography or capillary electrophoresis with electrophoresis with electrospray mass spectrometry. J Chromatogr A 910:69
Ramos ML, Tyson JF, Curran DJ (1998) Determination of acetaminophen by flow injection with online chemical derivatization: investigations using visible and FTIR spectrophotometry. Anal Chim Acta 364:107
Zouhair B, Salvador G, Miguel de la G (1996) Flow injection-fourier transform infrared spectrometric determination of paracetamol in pharmaceuticals. Analyst 121:635
Ivaska A, Ryan T (1981) Application of a voltammetric flow-through cell to flow-injection-analysis (FIA). Czech Chem Commun 46:2865
Lau OW, Luk SF, Cheung YM (1989) Simultaneous determination of ascorbic acid, caffeine and paracetamol in drug formulations by differential-pulse voltammetry using a glassy carbon electrode. Analyst 114:1047
Kunkel A, Günter S, Wätzig H (1997) Quantitation of acetaminophen and salicylic acid in plasma using capillary electrophoresis without sample pretreatment improvement of precision. J Chromatogr A 768:125
Zhao SL, Bai WL, Yuan HY, Xiao D (2006) Detection of paracetamol by capillary electrophoresis with chemiluminescence detection. Anal Chim Acta 559:195
Knochen M, Giglio J, Reis BF (2003) Flow-injection spectrophotometric determination of paracetamol in tablets and oral solutions. J Pharm Biomed Anal 33:191
Bloonfield MS (2002) A sensitive and rapid assay for 4-aminophenol in paracetamol drug and tablet formulation, by flow injection analysis with spectrophotometric detection. Talanta 580:1301
Masawat P, Liawruangrath S, Vaneesorn Y, Liawruangrath B (2002) Design and fabrication of a low-cost flow-through cell for the determination of acetaminophen in pharmaceutical formulations by flow injection cyclic voltammetry. Talanta 58:1221
Li QL (1995) Electroanalytical chemistry. Beijing Normal University Press, China, p 227
Acknowledgments
This study was supported by the National Natural Science Foundation of China (20575042, 20775050) and the Science Foundation of the Chinese Education Commission (105141)
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material
Table S1
Recovery test of acetaminophen sample with amperometric detection on FIA-GNMCPE (n = 5) (DOC 27.5 KB)
Fig. S1
Cyclic voltammograms of the GNMCPE in 50 mg L−1 acetaminophen in potential range between −0.50 and +1.0 V at different scan rate: (a) 0.05, (b) 0.1, (c) 0.2, (d) 0.3, (e) 0.4 V s−1, respectively. Inset: plot of peak current on the square root of the scan rates (DOC 406 KB)
Fig. S2
Observed dependence of current on pH for 0.1 mol L−1 acetaminophen (DOC 39.0 KB)
Fig. S3
Observed dependence of current on phosphate buffer solution concentration (mol L−1) (DOC 42.0 KB)
Fig. S4
Flow injection analysis with amperometric detection results for a GNMCPE in 2.5 mg L−1 acetaminophen, 0.1 mol L−1 phosphate buffer solution (DOC 221 KB)
Fig. S5
Effect of injection volume of sample on a GNMCPE in 2.5 mg L−1 acetaminophen, 0.1 mol L−1 phosphate buffer solution (DOC 42.0 KB)
Fig. S6
Effect of injection time on the magnitude of amperometric response at a GNMCPE in 2.5 mg L−1 acetaminophen, 0.1 mol L−1 phosphate buffer solution (DOC 138 KB)
Fig. S7
Effect of detection volume of flow-through cell for flow injection analysis at a GNMCPE in 2.5 mg L−1 acetaminophen, 0.1 mol L−1 phosphate buffer solution (DOC 74.5 KB)
Rights and permissions
About this article
Cite this article
Xu, Z., Yue, Q., Zhuang, Z. et al. Flow injection amperometric determination of acetaminophen at a gold nanoparticle modified carbon paste electrode. Microchim Acta 164, 387–393 (2009). https://doi.org/10.1007/s00604-008-0072-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-008-0072-8