Abstract
The authors describe the synthesis of a magnetic metal-organic framework (MOF) of type MIL-53(Fe) for coextraction of phenols and anilines from various environmental samples. A quick method for dispersive micro-solid phase extraction (D-μ-SPE) was developed for coextraction of the analytes 4-nitrophenol (4-NP), 4-chlorophenol (4-CP), 4-chloroaniline (4-CA), 1-amino-2-naphtol (1-A2N) and 2, 4-dichloroaniline (2, 4-DCA). The MOF was characterized by SEM, TEM, FT-IR, EDS, thermogravimetry, VSM and XRD. The method was optimized by response surface methodology combined with desirability function approach, specifically with respect to pH value of the sample, amount of sorbent, sorption time, salt concentration, sample volume, type and volume of the eluent, and elution time. Following elution with acetonitrile, the analytes were quantified by HPLC with photodiode array detection. Responses are linear in 0.1–2000 μg·L−1 concentration ranges. The limits of detection and relative standard deviations (for n = 5) are in the range of 0.03–0.2 μg·L−1 and 3.5–12.6%, respectively. Enrichment factors are 113, 61, 87, 144 and 114 for 4-NP, 4-CP, 4-CA, 1-A2N and 2,4-DCA, respectively. Recoveries from spiked samples ranged from 39.5 to 93.3%. The magnetic sorbent was successfully applied to the coextraction and determination of the analytes in river, rain and hookah water samples.

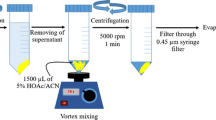
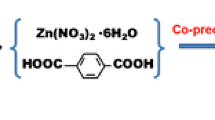
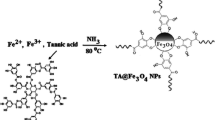
Schematic presentation for the synthesis of (a) Fe3O4 nanoparticles (NPs) and (b) Fe3O4@MIL-53(Fe). Fe3O4@MIL-53(Fe) was employed as a new nanosorbent in dispersive micro-solid phase extraction of phenols and anilines. The limits of detection are in the range of 0.03–0.2 μg·L−1.


Similar content being viewed by others
References
Young A, Lai G, Hung B, Yuen A, He Y (2011) Determination of trace Chloroanilines in environmental water samples using hollow Fiber-based liquid phase microextraction. Chromatographia 74:83–88. https://doi.org/10.1007/s10337-011-2022-6
Yang Q, Chen X, Jiang X (2013) Liquid–liquid microextraction of Nitrophenols using supramolecular solvent and their determination by HPLC with UV detection. Chromatographia 76:1641–1647. https://doi.org/10.1007/s10337-013-2554-z
Pan YL, Chen F, Zhang MY, Wang TQ, Xu ZC, Zhang W, Chu QC, Ye JN (2013) Sensitive determination of chloroanilines in water samples by hollow fiber-based liquid-phase microextraction prior to capillary electrophoresis with amperometric detection. Electrophoresis 34:1241–1248. https://doi.org/10.1002/elps.201200320
Jalilian N, Ebrahimzadeh H, Asgharinezhad AA (2017) Dispersive micro-solid phase extraction of aromatic amines based on an efficient sorbent made from poly(1,8-diaminonaphtalen) and magnetic multiwalled carbon nanotubes composite. J Chromatogr A 1499:38–47. https://doi.org/10.1016/j.chroma.2017.03.087
Babaee S, Daneshfar A (2016) Extraction of phenolic compounds from water samples by dispersive micro-solid-phase extraction. J Sep Sci 39:2508–2516. https://doi.org/10.1002/jssc.201500977
Sun XM, Sun Y, Wu LW, Jiang CZ, Yu X, Gao Y, Wang LY, Song DQ (2012) Development of a vortex-assisted ionic liquid microextraction method for the determination of aromatic amines in environmental water samples. Anal Methods 4:2074–2080. https://doi.org/10.1039/C2AY25056J
Baciocchi R, Attina M, Lombardi G, Boni MR (2001) Fast determination of phenols in contaminated soils. J Chromatogr A 911:135–141. https://doi.org/10.1016/S0021-9673(00)01249-8
Wennrich L, Popp P, Molder M (2000) Determination of chlorophenols in soils using accelerated solvent extraction combined with solid-phase microextraction. Anal Chem 72:546–551. https://doi.org/10.1021/ac990463r
Zhang WB, Xiao XM, An TC, Song ZG, Fu JM, Sheng GY, Cui MC (2003) Kinetics, degradation pathway and reaction mechanism of advanced oxidation of 4-nitrophenol in water by a UV/H2O2 process. J Chem Technol Biotechnol 78:788–794. https://doi.org/10.1002/jctb.864
Chung KT, Fulk GF, Egan M (1978) Reduction of azo dyes by intestinal anaerobes. Appl Environ Microbiol 35:558–562
Asgharinezhad AA, Ebrahimzadeh H (2016) A simple and fast method based on mixed hemimicelles coated magnetite nanoparticles for simultaneous extraction of acidic and basic pollutants. Anal Bioanal Chem 408:473–486. https://doi.org/10.1007/s00216-015-9114-3
Jauregui O, Galceran MT (1997) Determination of phenols in water by on-line solid-phase disk extraction and liquid chromatography with electrochemical detection. Anal Chim Acta 340:191–199. https://doi.org/10.1016/S0003-2670(96)00504-1
Cai MQ, Wei XQ, Du CH, Ma XM, Jin MC (2014) Novel amphiphilic polymeric ionic liquid-solid phase micro-extraction membrane for the preconcentration of aniline as degradation product of azo dye Orange G under sonication by liquid chromatography–tandem mass spectrometry. J Chromatogr A 1349:24–29. https://doi.org/10.1016/j.chroma.2014.05.008
Sarafraz-Yazdi A, Sayyar Ardaki M, Amiri A (2013) Determination of monocyclic aromatic amines using headspace solid-phase microextraction based on sol gel technique prior to GC. J Sep Sci 36:1629–1635. https://doi.org/10.1002/jssc.201200940
Zhu L, Lee HK (2001) Liquid–liquid–liquid microextraction of nitrophenols with a hollow fiber membrane prior to capillary liquid chromatography. J Chromatogr A 924:407–414. https://doi.org/10.1016/S0021-9673(01)00906-2
Zhang D, Zhang L, Liu T (2018) A magnetic cellulose-based carbon fiber hybrid as a dispersive solid-phase extraction material for the simultaneous detection of six bisphenol analogs from environmental samples. Analyst 143:3100–3106. https://doi.org/10.1039/C8AN00544C
Jalilian N, Ebrahimzadeh H, Asgharinezhad AA (2018) Determination of acidic, basic and amphoteric drugs in biological fluids and wastewater after their simultaneous dispersive micro-solid phase extraction using multiwalled carbon nanotubes/magnetite nanoparticles@poly(2-aminopyrimidine) composite. Microchem J 143:337–349. https://doi.org/10.1016/j.microc.2018.08.037
Chisvert A, Cardenas S, Lucena R (2019) Dispersive micro-solid phase extraction. TrAC Trends Anal Chem 112:226–233. https://doi.org/10.1016/j.trac.2018.12.005
Asgharinezhad AA, Jalilian N, Ebrahimzadeh H, Panjali Z (2015) A simple and fast method based on new magnetic ion imprinted polymer nanoparticles for the selective extraction of Ni(II) ions in different food samples. RSC Adv 5:45510–45519. https://doi.org/10.1039/C5RA05639J
Jalilian N, Ebrahimzadeh H, Asgharinezhad AA, Molaei K (2017) Extraction and determination of trace amounts of gold(III), palladium(II), platinum(II) and silver(I) with the aid of a magnetic nanosorbent made from Fe3O4-decorated and silica-coated graphene oxide modified with a polypyrrole-polythiophene copolymer. Microchim Acta 184:2191–2200. https://doi.org/10.1007/s00604-017-2170-y
Zhang H, Yuan Y, Sun Y, Niu C, Qiao F, Yan H (2018) An ionic liquid-magnetic graphene composite for magnet dispersive solid-phase extraction of triazine herbicides in surface water followed by high performance liquid chromatography. Analyst 143:175–181. https://doi.org/10.1039/C7AN01290J
Yang Z, Xu X, Liang X, Lei C, Wei Y, He P, Lv B, Ma H, Lei Z (2016) MIL-53(Fe)-graphene nanocomposites: efficient visible-light photocatalysts for the selective oxidation of alcohols. Appl Catal B 198:112–123. https://doi.org/10.1016/j.apcatb.2016.05.041
Hashemi B, Zohrabi P, Raza N, Kim KH (2017) Metal-organic frameworks as advanced sorbents for the extraction and determination of pollutants from environmental, biological, and food media. TrAC Trends Anal Chem 97:65–82. https://doi.org/10.1016/j.trac.2017.08.015
Rocio-Bautista P, Gonzalez-Hernandez P, Pino V, Pasan J, Afonso AM (2017) Metal-organic frameworks as novel sorbents in dispersive-based microextraction approaches. TrAC Trends Anal Chem 90:114–134. https://doi.org/10.1016/j.trac.2017.03.002
Ghorbani-Kalhor E (2016) A metal-organic framework nanocomposite made from functionalized magnetite nanoparticles and HKUST-1 (MOF-199) for preconcentration of Cd(II), Pb(II), and Ni(II). Microchim Acta 183:2639–2647. https://doi.org/10.1007/s00604-016-1896-2
Azizi Vahed T, Naimi-Jamal MR, Panahi L (2018) (Fe)MIL−100-met@alginate: a hybrid polymer–MOF for enhancement of metformin's bioavailability and pH-controlled release. New J Chem 42:11137–11146. https://doi.org/10.1039/C8NJ01946K
Araya T, Jia M, Yang J, Zhao P, Cai K, Ma W, Huang Y (2017) Resin modified MIL-53(Fe) MOF for improvement of photocatalytic performance. Appl Catal B 203:768–777. https://doi.org/10.1016/j.apcatb.2016.10.072
Rezvani M, Asgharinezhad AA, Ebrahimzadeh H, Shekari N (2014) A polyaniline-magnetite nanocomposite as an anion exchange sorbent for solid-phase extraction of chromium(VI) ions. Microchim Acta 181:1887–1895. https://doi.org/10.1007/s00604-014-1262-1
Banerjee A, Gokhale R, Bhatnagar S, Jog J, Bhardwaj M, Lefez B, Hannoyerc B, Ogale S (2012) MOF derived porous carbon–Fe3O4 nanocomposite as a high performance, recyclable environmental superadsorbent. J Mater Chem 22:19694–19699. https://doi.org/10.1039/C2JM33798C
Madrakian T, Afkhami A, Zadpour B, Ahmadi M (2015) New synthetic mercaptoethylamino homopolymer-modified maghemite nanoparticles for effective removal of some heavy metal ions from aqueous solution. J Ind Eng Chem 21:1160–1166. https://doi.org/10.1016/j.jiec.2014.05.029
Asgharinezhad AA, Ebrahimzadeh H (2015) Coextraction of acidic, basic and amphiprotic pollutants using multiwalled carbon nanotubes/magnetite nanoparticles@polypyrrole composite. J Chromatogr A 1412:1–11. https://doi.org/10.1016/j.chroma.2015.07.087
Asiabi H, Yamini Y, Rezaei F, Seidi S (2016) Nanostructured polypyrrole for automated and electrochemically controlled in-tube solid-phase microextraction of cationic nitrogen compounds. Microchim Acta 183:1449–1458. https://doi.org/10.1007/s00604-015-1534-4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 997 kb)
Rights and permissions
About this article
Cite this article
Jalilian, N., Ebrahimzadeh, H. & Asgharinezhad, A.A. A nanosized magnetic metal-organic framework of type MIL-53(Fe) as an efficient sorbent for coextraction of phenols and anilines prior to their quantitation by HPLC. Microchim Acta 186, 597 (2019). https://doi.org/10.1007/s00604-019-3698-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3698-9