Abstract
Monodisperse porous silica microspheres were functionalized with the iminodiacetic acid/copper(II) complex and then evaluated as a group-specific peroxidase-mimicking nanozyme for colorimetric determination of histidine-tagged (His-tagged) proteins. The green fluorescent protein (GFP) was selected as a typical His-tagged protein. The specificity for GFP and the peroxidase-like activity for the selected substrate were obtained by immobilizing the complex on the porous microspheres. The modified microspheres were also evaluated as a group specific immobilized metal affinity chromatography (IMAC) sorbent for the purification of GFP from Escherichia coli extract. The peroxidase-like activity of the microspheres was inhibited by the GFP adsorbed onto the microspheres due to the interaction of His-tagged protein with the immobilized Cu(II) complex. Ortho-phenylenediamine is used as a substrate for the enzyme mimic. The photometric response (measured at 416 nm) is linear in the 9.0–92 μg·mL−1 GFP concentration range in E. coli lysate. The limit of detection is 6.9 μg·mL−1.

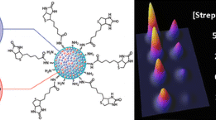
Schematic representation of metal affinity chromatography-based colorimetric determination of histidine-tagged proteins using silica microspheres functionalized with iminodiacteic acid/copper (II) complex as a peroxidase mimic.






Similar content being viewed by others
References
Mu JS, He Y, Wang Y (2016) Copper-incorporated SBA-15 with peroxidase-like activity and its application for colorimetric detection of glucose in human serum. Talanta 148:22–28. https://doi.org/10.1016/j.talanta.2015.10.060
Zhao TT, Jiang ZW, Zhen SJ, Huang CZ, Li YF (2019) A copper(II)/cobalt(II) organic gel with enhanced peroxidase-like activity for fluorometric determination of hydrogen peroxide and glucose. Microchim Acta 186:168. https://doi.org/10.1007/s00604-019-3290-3
Shi BF, Su YB, Duan Y, Chen SY, Zuo WY (2019) A nanocomposite prepared from copper(II) and nitrogen-doped graphene quantum dots with peroxidase mimicking properties for chemiluminescent determination of uric acid. Microchim Acta 186:397–310. https://doi.org/10.1007/s00604-019-3491-9
Zhu JL, Nie W, Wang Q, Li JW, Li H, Wen W, Bao T, Xiong HY, Zhang XH, Wang SF (2018) In situ growth of copper oxide-graphite carbon nitride nanocomposites with peroxidase-mimicking activity for electrocatalytic and colorimetric detection of hydrogen peroxide. Carbon 129:29–37. https://doi.org/10.1016/j.carbon.2017.11.096
Xu SJ, Wang YQ, Zhou DY, Kuang M, Fang D, Yang WH, Wei SJ, Ma L (2016) A novel chemiluminescence sensor for sensitive detection of cholesterol based on the peroxidase-like activity of copper nanoclusters. Sci Rep 6:39157. https://doi.org/10.1038/srep39157
Tian L, Qi JX, Oderinde O, Yao C, Song W, Wang YH (2018) Planar intercalated copper (II) complex molecule as small molecule enzyme mimic combined with Fe3O4 nanozyme for bienzyme synergistic catalysis applied to the microRNA biosensor. Biosens Bioelectron 110:110–117. https://doi.org/10.1016/j.bios.2018.03.045
Wu CW, Harroun SG, Lien CW, Chang HT, Unnikrishnan B, Lai IPJ, Chang JY, Huang CC (2016) Self-templated formation of aptamer-functionalized copper oxide nanorods with intrinsic peroxidase catalytic activity for protein and tumor cell detection. Sensors Actuators B Chem 227:100–107. https://doi.org/10.1016/j.snb.2015.12.045
Wang CH, Gao J, Cao YL, Tan HL (2018) Colorimetric logic gate for alkaline phosphatase based on copper (II)-based metal-organic frameworks with peroxidase-like activity. Anal Chim Acta 1004:74–81. https://doi.org/10.1016/j.aca.2017.11.078
Chang YQ, Zhang Z, Hao JH, Yang WS, Tang JL (2016) A simple label free colorimetric method for glyphosate detection based on the inhibition of peroxidase-like activity of cu(II). Sensors Actuators B Chem 228:410–415. https://doi.org/10.1016/j.snb.2016.01.048
Zheng AX, Zhang XL, Gao J, Liu XL, Liu JF (2016) Peroxidase-like catalytic activity of copper ions and its application for highly sensitive detection of glypican-3. Anal Chim Acta 941:87–93. https://doi.org/10.1016/j.aca.2016.08.036
Wang HB, Li Y, Dong GL, Gan T, Liu YM (2017) A convenient and label-free colorimetric assay for dopamine detection based on the inhibition of the cu(II)-catalyzed oxidation of a 3,3′,5,5′-tetramethylbenzidine-H2O2 system. New J Chem 41:14364–14369. https://doi.org/10.1039/c7nj02710a
Singh P, Nath P, Arun RK, Mandal S, Chanda N (2016) Novel synthesis of a mixed cu/CuO-reduced graphene oxide nanocomposite with enhanced peroxidase-like catalytic activity for easy detection of glutathione in solution and using a paper strip. RSC Adv 6:92729–92738. https://doi.org/10.1039/c6ra20882g
Wang SQ, Deng WF, Yang L, Tan YM, Xie QJ, Yao SZ (2017) Copper-based metal organic framework nanoparticles with peroxidase-like activity for sensitive colorimetric detection of staphylococcus aureus. ACS Appl Mater Interfaces 9:24440–24445. https://doi.org/10.1021/acsami.7b07307
Thawari AG, Rao CP (2016) Peroxidase-like catalytic activity of copper-mediated protein-inorganic hybrid nanoflowers and nanofibers of beta-lactoglobulin and alpha-lactalbumin: synthesis, spectral characterization, microscopic features, and catalytic activity. ACS Appl Mater Interfaces 8:10392–10402. https://doi.org/10.1021/acsami.5b12591
Xiong YH, Qin YM, Su LJ, Ye FG (2017) Bioinspired synthesis of Cu2+−modified covalent triazine framework: a new highly efficient and promising peroxidase mimic. Chem Eur J 23:11037–11045. https://doi.org/10.1002/chem.201701513
Qin Y, Zhang Q, Li YD, Liu XL, Lu ZX, Zheng LY, Liu SX, Cao QE, Ding ZT (2017) Copper metal-organic polyhedra nanorods with high intrinsic peroxidase-like activity at physiological pH for bio-sensing. J Mater Chem B 5:9365–9370. https://doi.org/10.1039/c7tb02388j
Yin YQ, Gao CL, Xiao Q, Lin G, Lin Z, Cai ZW, Yang HH (2016) Protein-metal organic framework hybrid composites with intrinsic peroxidase-like activity as a colorimetric biosensing platform. ACS Appl Mater Interfaces 8:29052–29061. https://doi.org/10.1021/acsami.6b09893
Kulsharova G, Dimov N, Marques MPC, Szita N, Baganz F (2018) Simplified immobilisation method for histidine-tagged enzymes in poly(methyl methacrylate) microfluidic devices. New Biotechnol 47:31–38. https://doi.org/10.1016/j.nbt.2017.12.004
Salimi K, Usta DD, Kocer I, Celik E, Tuncel A (2017) Highly selective magnetic affinity purification of histidine-tagged proteins by Ni2+ carrying monodisperse composite microspheres. RSC Adv 7:8718–8726. https://doi.org/10.1039/c6ra27736e
Wang JJ, Zhang R, Yang XX, Liu XY, Zhang HX (2018) Facile synthesis of copper(II)-decorated functional mesoporous material for specific adsorption of histidine-rich proteins. Talanta 176:308–317. https://doi.org/10.1016/j.talanta.2017.08.016
Schwaminger SP, Fraga-Garcia P, Blank-Shim SA, Straub T, Haslbeck M, Muraca F, Dawson KA, Berensmeier S (2019) Magnetic one-step purification of his-tagged protein by bare iron oxide nanoparticles. ACS Omega 4:3790–3799. https://doi.org/10.1021/acsomega.8b03348
Chang MM, Qin Q, Wang BH, Xia T, Lv WJ, Sun XS, Shi XZ, Xu GW (2019) Carboxymethylated polyethylenimine modified magnetic nanoparticles specifically for purification of his-tagged protein. J Sep Sci 42:744–753. https://doi.org/10.1002/jssc.201800969
Joshi PN, Rai V (2019) Single-site labeling of histidine in proteins, on-demand reversibility, and traceless metal-free protein purification. Chem Commun 55:1100–1103. https://doi.org/10.1039/c8cc08733d
Xu L, Wang R, Cao HY, Xu T, Han LL, Huang CD, Jia LY (2019) A facile method to oriented immobilization of his-tagged BirA on Co3+-NTA agarose beads. Enzym Microb Technol 120:36–42. https://doi.org/10.1016/j.enzmictec.2018.09.004
Kip C, Tosun RB, Alpaslan S, Kocer I, Celik E, Tuncel A (2019) Ni(II)-decorated porous titania microspheres as a stationary phase for column chromatography applications: highly selective purification of hemoglobin from human blood. Talanta 200:100–106. https://doi.org/10.1016/j.talanta.2019.03.045
Mishra A, Ravikumar S, Song YH, Prabhu NS, Kim H, Hong SH, Cheon S, Noh J, Chi KW (2014) A new arene-Ru based supramolecular coordination complex for efficient binding and selective sensing of green fluorescent protein. Dalton Trans 43:6032–6040. https://doi.org/10.1039/c3dt53186d
Colwell M, Ahmed N, Butkowski R (2017) Detection of histidine-rich glycoprotein and fibrinogen with nickel-enzyme conjugates: purification of rabbit HRG. Anal Biochem 525:67–72. https://doi.org/10.1016/j.ab.2017.02.013
Kip C, Gulusur H, Celik E, Usta DD, Tuncel A (2019) Isolation of RNA and beta-NAD by phenylboronic acid functionalized, monodisperse-porous silica microspheres as sorbent in batch and microfluidic boronate affinity systems. Colloids Surf B: Biointerfaces 174:333–342. https://doi.org/10.1016/j.colsurfb.2018.11.012
Ogut E, Kip C, Gokcal B, Tuncel A (2019) Aggregation-resistant nanozyme containing accessible magnetite nanoparticles immobilized in monodisperse-porous silica microspheres for colorimetric assay of human genomic DNA. J Colloid Interface Sci 550:90–98. https://doi.org/10.1016/10.1016/j.jcis.2019.04.089
Gunal G, Kip C, Ogut SE, Usta DD, Senlik E, Kibar G, Tuncel A (2017) Human genomic DNA isolation from whole blood using a simple microfluidic system with silica- and polymer-based stationary phases. Mater Sci Eng C Mater Biol Appl 74:10–20. https://doi.org/10.1016/j.msec.2016.12.118
Svendsen A, Kiefer HV, Pedersen HB, Bochenkova AV, Andersen LH (2017) Origin of the intrinsic fluorescence of the green fluorescent protein. J Am Chem Soc 139:8766–8771. https://doi.org/10.1021/jacs.7b04987
Peterffy J, Szabo M, Szilagyi L, Lanyi S, Abraham B (2015) Fluorescence of a histidine-modified enhanced green fluorescent protein (EGFP) effectively quenched by copper(II) ions. Part II Molecular determinants. J Fluoresc 25:871–883. https://doi.org/10.1007/s10895-015-1567-4
Zhang ZJ, Zhang XH, Liu BW, Liu JW (2017) Molecular imprinting on inorganic nanozymes for hundred-fold enzyme specificity. J Am Chem Soc 139:5412–5419. https://doi.org/10.1021/jacs.7b00601
Zhang ZJ, Liu BW, Liu JW (2017) Molecular imprinting for substrate selectivity and enhanced activity of enzyme mimics. Small 13:1602730. https://doi.org/10.1002/smll.201602730
Zhang ZJ, Li YQ, Zhang XH, Liu JW (2019) Molecularly imprinted nanozymes with faster catalytic activity and better specificity. Nanoscale 11:4854–4863. https://doi.org/10.1039/c8nr09816f
Acknowledgements
This research was supported by Hacettepe University Scientific Research Projects Coordination Unit under contract numbered as FBA-2019-17337. Special thanks are extended to Turkish Academy of Sciences (TUBA) for the research support provided to Dr. Ali Tuncel as a full-member. Council of Higher Education of Turkey is acknowledged for providing the CoHE-100/2000 Doctoral Scholarship to Dilek Şahinbaş.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1015 kb)
Rights and permissions
About this article
Cite this article
Gökçal, B., Kip, Ç., Şahinbaş, D. et al. Silica microspheres functionalized with the iminodiacetic acid/copper(II) complex as a peroxidase mimic for use in metal affinity chromatography-based colorimetric determination of histidine-tagged proteins. Microchim Acta 187, 121 (2020). https://doi.org/10.1007/s00604-019-4087-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-4087-0