Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) is a pathogen of great economic significance that impacts the swine industry globally. Since the first report of a porcine reproductive and respiratory syndrome (PRRS) outbreak, tremendous efforts to control this disease, including various national policies and plans incorporating the use of multiple modified live-virus vaccines, have been made. However, PRRSV is still a significant threat to the swine industry, and new variants continually emerge as a result of PRRSV evolution. Several studies have shown that pandemic PRRSV strains have enormous genetic diversity and that commercial vaccines can only provide partial protection against these strains. Therefore, effective anti-PRRSV drugs may be more suitable and reliable for PRRSV control. In this study, we observed that isobavachalcone (IBC), which was first isolated from Psoralea corylifolia, had potent anti-PRRSV activity in vitro. Although many biological activities of IBC have been reported, this is the first report describing the antiviral activity of IBC. Furthermore, after a systematic investigation, we demonstrated that IBC inhibits PRRSV replication at the post-entry stage of PRRSV infection. Thus, IBC may be a candidate for further evaluation as a therapeutic agent against PRRSV infection of swine in vivo.
Similar content being viewed by others
Introduction
Porcine reproductive and respiratory syndrome virus (PRRSV) is a positive-strand RNA virus that is approximately 15 kb in length and belongs to the family Arteriviridae [5]. PRRSV is one of the most important viruses hindering the development of the swine industry globally, especially with the emergence of highly pathogenic PRRSV (HP-PRRSV) [5, 38]. Recently, new PRRSV mutant strains have become pandemic in China [1, 16, 17, 35, 37, 39] and are named NADC30-like PRRSV strains for their genomic similarity to the NADC30 strain isolated in the US in 2008 [3]. NADC30-like PRRSV strains feature different recombinations and have produced many mosaic isolates, enhancing their genetic diversity. Currently, the most effective methods to control viral diseases are vaccines and antiviral agents. However, vaccination against PRRSV infection has achieved limited success, primarily due to the high genetic diversity of the virus [9, 10, 22, 29]. In addition to genetic mutation, PRRSV genetic diversity is also generated by recombination among different strains, including vaccine strains [7, 18, 23, 30, 33, 37]. Importantly, commercial vaccines cannot provide complete protection against heterologous PRRSV infection [1, 21]. The problems outlined above indicate that the use of vaccines may not be an ideal strategy for PRRSV control; however, exploring effective anti-PRRSV drugs may overcome these issues.
Isobavachalcone (IBC) is a prenylated chalcone of the flavonoid subclass that was first isolated from Psoralea corylifolia in 1968 [14]. IBC possesses a wide spectrum of biological activities, including antibacterial, antifungal, anticancer, anti-reverse-transcriptase, antitubercular and antioxidant functions [14]. However, whether IBC has potential antiviral activity remains unclear. In the current study, for the first time, we observed that IBC has anti-PRRSV activity. Furthermore, after a systematic investigation, we demonstrated that IBC inhibits PRRSV replication at the post-entry stage of PRRSV infection.
Materials and methods
Cell, viruses and inhibitors
Porcine alveolar macrophages (PAMs) were obtained from the lungs of 4-week-old specific-pathogen-free (SPF) piglets, and monkey kidney cells (Marc145) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS). The highly pathogenic PRRSV strain HuN4 [25, 26] and the NADC30-like PRRSV strain HeN-L1 (isolated in our lab) were propagated and titrated in Marc145 cells. IBC was purchased from Shanghai Tauto Biotech Company and was dissolved in ethanol and used at the concentrations indicated.
Determination of isobavachalcone cytotoxicity
The cytotoxicity of IBC was evaluated for PAMs and Marc145 cells. Briefly, twofold dilutions of IBC from a starting concentration of 25.6 μM were applied to 80% confluent cells in a 96-well plate. After incubation for 24 h at 37 °C, the cytotoxicity of IBC was evaluated using a Cell Counting Kit-8 (CCK8, Dojindo Laboratories, Japan) according to manufacturer’s protocol. The CC50 was defined as the concentration of IBC that reduced the absorbance of treated cells by 50% relative to that of the cell control.
Antiviral activity of IBC against PRRSV infection
A total of 3 × 106 Marc145 cells were plated in a 6-well plate, and 18 h later, the cells were infected with HuN4 (multiplicity of infection [MOI] = 1) for 2 h at 37 °C. Next, the culture medium was replaced with different concentrations of IBC diluted in DMEM containing 2% FBS. Cells were harvested at the indicated times for further Western blot or immunofluorescence analysis.
RNA extraction and absolute quantitative real-time PCR (RT-PCR) analysis
The total RNA of all samples was extracted using an RNeasy Plus Mini Kit (QIAGEN, Germany), and 1 μg of total RNA was reverse transcribed into cDNA according to the manufacturer’s protocol (PrimeScript™ RT Reagent Kit with gDNA Eraser, TaKaRa). The real-time PCR procedure was performed using an Agilent Mx3005P Real-Time PCR System. The primers and probes were described in a previous report [28].
Immunofluorescence
Cells were infected with HuN4 for 2 h and were then treated with various concentrations of IBC. After incubating for the indicated time, cells were fixed in 95% ethanol, permeabilized with 0.5% saponin in PBS, and then blocked with 5% bovine serum albumin (BSA) for 1 h at room temperature (RT). After blocking, the cells were incubated with a mouse anti-PRRSV N protein monoclonal antibody (IgG2b, made in our lab) at a dilution of 1:500 for 1 h at RT, and dsRNA was stained with the mouse monoclonal antibody J2 (Scicons, Hungary) at a dilution of 1:200 for 1 h at RT. After washing, an aliquot of 1:100-diluted FITC-labeled anti-mouse IgG (Sigma) was used as the secondary antibody. For nuclear visualization, cells were treated with DAPI (Sigma). Immunofluorescence was observed using a Leica TCS SP5 confocal microscope.
Western blotting analysis
Cells were washed twice with PBS and then prepared with RIPA lysis buffer (Solarbio). The cell lysates were centrifuged at 12,000 rpm for 5 min at 4 °C. The supernatants were collected, and protein concentrations were determined by BCA assay. Next, proteins from each sample were separated via 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), after which the protein bands were transferred onto a polypropylene fluoride (PVDF) membrane. The membrane was blocked with 5% nonfat milk for 2 h at RT and then incubated with mouse anti-PRRSV N protein monoclonal antibody (1:3000, made in our laboratory) or anti-β-actin antibody (1:4000, Sigma) for 1.5 h at RT. After being washed three times with PBST (0.05% Tween 20 in PBS), the membrane was incubated for 1 h with HRP-conjugated anti-mouse IgG antibody (Sigma). Protein bands were visualized with ECL chemiluminescence reagent (Thermo Scientific). Western blot bands were quantified and analyzed using ImageJ. The relative intensity ratio of protein bands (N protein/β-actin) was calculated as an indicator of PRRSV replication ability, with the value of the control group set as 1; other IBC treated groups were normalized correspondingly.
Determination of the IC50 of IBC against PRRSV infection
To further investigate the anti-PRRSV activity of IBC, the drug concentration that could inhibit PRRSV infections by approximately 50% (50% inhibitory concentration, IC50) was determined. Marc145 cells were infected with PRRSV (MOI = 1) for 2 h at 37 °C and then treated with twofold dilutions of IBC. Total RNA was isolated from cells at 24 h postinfection (p.i.), and intracellular PRRSV RNA copy numbers were determined by RT-PCR. The percentage inhibition was calculated as [(RNA copies in control cells - (RNA copies in cells treated with IBC)] ÷ (RNA copies of the control) × 100 at the indicated IBC concentration, and the value for the control group was set as 1. The other IBC-treated groups were normalized correspondingly.
Attachment and entry assay
A virus attachment and entry assay was performed by pre-incubating Marc145 cells at 4 °C for 30 min, after which PRRSV HuN4 (MOI = 1) and 5 μM IBC were added, and the cells were incubated for an additional 2 h at 4 °C to allow attachment of the virus to the cells. Next, the cells were washed three times with cold PBS to remove unbound virus. The cells were then collected and either used to detect PRRSV RNA levels via real-time PCR (to assess viral attachment) or provided fresh medium containing 5 μM IBC and incubated at 37 °C for 2 h. A temperature shift from 4 to 37 °C was performed to promote entry of the virus into the cells, and after a 2-h incubation, total RNA was collected and analyzed by real-time PCR (to assess viral entry).
Time-of-addition assay with isobavachalcone
Marc145 cells were infected with PRRSV HuN4 (MOI = 1) at 37 °C. After a 2-h incubation, cells were washed three times with PBS to remove unbound viruses and were replenished with 2% FBS. For the postinfection inhibition study, HuN4-infected cells were replenished with 2% FBS supplemented with 5 or 10 μM IBC at specific time points (2, 6, 12, or 24 h). Cells were harvested at 24 h after IBC treatment, and the levels of PRRSV replication were estimated by Western blot analysis.
Statistical analysis
Origin 8.0 software was used for all graphical representations. The IC50 was calculated using non-linear regression. Statistical significance was established using an independent t-test, and P-values less than 0.05 were considered significant.
Results
Isobavachalcone inhibits PRRSV replication in vitro
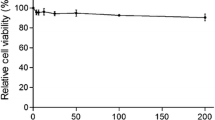
The structure of IBC is shown in Figure 1A. To assess the anti-PRRSV activity of IBC, we first evaluated the cytotoxicity of IBC on Marc145 cells (Fig. 1B) and PAM cells (Fig. 1C), and the results indicated that cell viability was not significantly affected by IBC at concentrations up to 25.6 μM and that the CC50 of IBC for Marc145 cells was 63.09 μM (data not show). The cytotoxic effect of the IBC solvent (alcohol) was also evaluated, the results of which indicated that the alcohol had no effect on Marc145 cells (Fig. 1B) and PAM cells (Fig. 1C). Next, we evaluated the anti-PRRSV capabilities of IBC in Marc145 cells that were infected with PRRSV HuN4 (MOI = 1) and then treated with different concentrations of IBC. We first determined progeny virus titers and found that IBC significantly inhibited PRRSV replication in a dose-dependent manner (Fig. 1D). To confirm this finding, we also performed an indirect immunofluorescence assay by staining cells with a PRRSV N protein antibody and then observed PRRSV replication by fluorescence microscopy. As shown in Figure 1E, PRRSV replication was significantly blocked by IBC. Western blot analysis revealed that PRRSV N protein levels decreased markedly, indicating that increasing concentrations of IBC significantly inhibited PRRSV replication (Fig. 1F). To determine whether this inhibition was strain specific, we additionally assayed a currently pandemic NADC30-like strain (HeN-L1) that was isolated in our lab and observed it also to be inhibited by IBC (Fig. 1G). Thus, these data indicate that IBC inhibition of PRRSV is not strain specific. Furthermore, the antiviral activity of IBC against PRRSV HuN4 was both dose dependent and successful, with an IC50 of 3.12 μM (Fig. 2) and a selectivity index (SI; the ratio of the CC50 to the IC50) of 22.02.
Isobavachalcone inhibits PRRSV replication. (A) Chemical structure of IBC. Cytotoxicity of IBC in (B) Marc145 cells and (C) PAM cells. (D) Virus titers of PRRSV at different IBC concentrations. (E) Inhibition of PRRSV infection by IBC as shown by IFA. (F) PRRSV HuN4 and (G) HeN-L1 inhibition by IBC as detected by Western blot analysis. Error bars represent the standard error of the mean
IBC inhibits PRRSV at an early stage
Despite the evidence that IBC inhibits PRRSV replication, the viral stage influenced by IBC remained unknown. To determine which step(s) in the viral life cycle are affected by IBC, we first performed time-of-drug-addition assays. The results indicated that IBC markedly inhibited PRRSV when added early after infection and remained inhibitory when added up to 6 h p.i., whereas PRRSV inhibition was limited when IBC was added late, at 12 h p.i. (Fig. 3). These data suggest that IBC targets the early phase of the viral life cycle, as it was not able to prevent viral infection after the virus entered the post-replicative stages of infection.
IBC does not influence PRRSV attachment and entry
Next, we tested whether IBC inhibited PRRSV by disturbing PRRSV attachment or entry. The results demonstrated that PRRSV attachment was not influenced by IBC (Fig. 4A). Interestingly, the PRRSV entry assay results were the same as those of the attachment assay, demonstrating that neither PRRSV attachment nor entry was influenced by IBC (Fig. 4B).
Viral RNA synthesis is considerably disrupted by isobavachalcone
The addition of IBC was inhibitory from 2 to 6 h p.i., whereas it had no effect on PRRSV attachment or entry (Fig. 4A and B). This inhibition at post-entry stages led us to speculate that IBC inhibits PRRSV replication at the stage of viral RNA synthesis. To test whether IBC blocked viral RNA replication, we performed inhibition experiments using PAM cells and stained for dsRNAs, which are intermediates in viral replication, with the J2 antibody, which recognizes dsRNA. The results indicated that dsRNA levels were significantly decreased in IBC-treated cells (Fig. 5A and B). These data clearly suggest that IBC blocks the early stages of the viral life cycle, most likely at the initiation of viral RNA replication.
Isobavachalcone inhibits PRRSV RNA synthesis. (A) PRRSV RNA synthesis was detected using a J2 antibody, which recognizes dsRNA, and cell nuclei were stained with DAPI. (B) Four fields were randomly selected for statistical analysis. The mean rates of positive cells among the total cells in each group were calculated using ImageJ
Discussion
Currently, PRRSV is a significant problem for the swine industry in China and was a veritable Pandora’s Box for the Chinese pig industry during the first outbreak [17]. The current pandemic strains are highly diverse, and commercial vaccines can provide only partial protection against these strains [27, 40]. Thus, anti-PRRSV drugs may be more suitable for PRRSV control in the future. Traditional Chinese medicine (TCM) has been widely used as a source of novel drugs, and many crude TCM herbal extracts have been shown to inhibit PRRSV replication [6, 24, 36]. IBC is extracted from P. corylifolia, which itself is used in TCM [14]. Cheng et al. systematically analyzed seventeen compounds derived from TCMs and tested their PRRSV antiviral activity in vitro [4]. Two compounds, chlorogenic acid and scutellarin, were shown to have great anti-PRRSV replication potential in their study, and the inhibition ratios of chlorogenic acid and scutellarin can reach 90.8 and 61.1%, respectively, at the maximum non-cytotoxic concentrations [4]; in contrast, the IBC inhibition ratio exceeded 90% at 15 μM, an improvement over the maximum non-cytotoxic concentrations for the other tested compounds. This result indicated that IBC is more effective than chlorogenic acid and scutellarin.
IBC has multiple biological activities; it potently abrogates Akt and Erk signaling pathways and exerts anti-proliferative effects on several human cancer cell lines, and it also induces apoptosis associated with the mitochondrial pathway [11, 12, 14]. Akt has been reported to play a positive role in PRRSV gene expression and entry, and PRRSV replication may be dependent on the activation of PI3K/Akt [19, 31, 32, 34]. The ERK signaling pathway has been demonstrated to play an important role in the post-entry steps of the PRRSV replication cycle and to enhance PRRSV infection [15]. The ERK pathway may also contribute to HP-PRRSV pathogenesis [2]. Apoptosis also influences PRRSV replication, since the induction of apoptosis in Marc145 cells increases virus replication [8]. IBC inhibition of PRRSV replication may occur through modulation of the above-mentioned pathway. IBC may be applicable as an efficacious and safe drug for the treatment of some cancers, such as neuroblastoma [20]. IBC has been reported to inhibit SARS-CoV papain-like protease; however, whether IBC inhibits infectious virus was not tested in that study [13]. This study is the first to report that IBC has potent antiviral activity, and we demonstrate here that it blocks PRRSV replication at the initiation of viral RNA replication in vitro. However, there are some unanswered questions that should be further explored in the future. First, whether IBC also shows anti-PRRSV activity in vivo must be investigated. Second, is the blockage of viral RNA formation a direct or indirect effect? Third, are there other viruses that are also inhibited by IBC?
In conclusion, we evaluated whether IBC has antiviral potential as a PRRSV infection treatment. The in vitro study established that IBC efficiently inhibited the replication of both HP-PRRSV and the current Chinese epidemic NADC30-like strains without significant drug toxicity. Thus, IBC may be a candidate for further evaluation as a therapeutic agent against PRRSV infection of swine in vivo.
References
Bai X, Wang Y, Xu X, Sun Z, Xiao Y, Ji G, Li Y, Tan F, Li X, Tian K (2016) Commercial vaccines provide limited protection to NADC30-like PRRSV infection. Vaccine 34:5540–5545
Bi Y, Guo XK, Zhao H, Gao L, Wang L, Tang J, Feng WH (2014) Highly pathogenic porcine reproductive and respiratory syndrome virus induces prostaglandin E2 production through cyclooxygenase 1, which is dependent on the ERK1/2-p-C/EBP-beta pathway. J Virol 88:2810–2820
Brockmeier SL, Loving CL, Vorwald AC, Kehrli ME Jr, Baker RB, Nicholson TL, Lager KM, Miller LC, Faaberg KS (2012) Genomic sequence and virulence comparison of four Type 2 porcine reproductive and respiratory syndrome virus strains. Virus Res 169:212–221
Cheng J, Sun N, Zhao X, Niu L, Song M, Sun Y, Jiang J, Guo J, Bai Y, He J, Li H (2013) In vitro screening for compounds derived from traditional chinese medicines with antiviral activities against porcine reproductive and respiratory syndrome virus. J Microbiol Biotechnol 23:1076–1083
Dokland T (2010) The structural biology of PRRSV. Virus Res 154:86–97
Du T, Nan Y, Xiao S, Zhao Q, Zhou EM (2017) Antiviral strategies against PRRSV infection. Trends Microbiol 25:968–979
Franzo G, Cecchinato M, Martini M, Ceglie L, Gigli A, Drigo M (2014) Observation of high recombination occurrence of Porcine Reproductive and Respiratory Syndrome Virus in field condition. Virus Res 194:159–166
Ge M, Zhang Y, Liu Y, Liu T, Zeng F (2016) Propagation of field highly pathogenic porcine reproductive and respiratory syndrome virus in MARC-145 cells is promoted by cell apoptosis. Virus Res 213:322–331
Hu J, Zhang C (2014) Porcine reproductive and respiratory syndrome virus vaccines: current status and strategies to a universal vaccine. Transbound Emerg Dis 61:109–120
Huang YW, Meng XJ (2010) Novel strategies and approaches to develop the next generation of vaccines against porcine reproductive and respiratory syndrome virus (PRRSV). Virus Res 154:141–149
Jin X, Shi YI (2016) Isobavachalcone induces the apoptosis of gastric cancer cells via inhibition of the Akt and Erk pathways. Exp Ther Med 11:403–408
Jing H, Zhou X, Dong X, Cao J, Zhu H, Lou J, Hu Y, He Q, Yang B (2010) Abrogation of Akt signaling by Isobavachalcone contributes to its anti-proliferative effects towards human cancer cells. Cancer Lett 294:167–177
Kim DW, Seo KH, Curtis-Long MJ, Oh KY, Oh JW, Cho JK, Lee KH, Park KH (2014) Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J Enzym Inhib Med Chem 29:59–63
Kuete V, Sandjo LP (2012) Isobavachalcone: an overview. Chin J Integr Med 18:543–547
Lee YJ, Lee C (2010) Porcine reproductive and respiratory syndrome virus replication is suppressed by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. Virus Res 152:50–58
Li C, Zhuang J, Wang J, Han L, Sun Z, Xiao Y, Ji G, Li Y, Tan F, Li X, Tian K (2016) Outbreak investigation of NADC30-like PRRSV in south-east China. Transbound Emerg Dis 63:474–479
Li X, Bao H, Wang Y, Tian K (2017) Widespread of NADC30-like PRRSV in China: another Pandora’s box for Chinese pig industry as the outbreak of highly pathogenic PRRSV in 2006? Infect Genet Evol 49:12–13
Liu J, Zhou X, Zhai J, Wei C, Dai A, Yang X, Luo M (2017) Recombination in JXA1-R vaccine and NADC30-like strain of porcine reproductive and respiratory syndrome viruses. Vet Microbiol 204:110–120
Ni B, Wen LB, Wang R, Hao HP, Huan CC, Wang X, Huang L, Miao JF, Fan HJ, Mao X (2015) The involvement of FAK-PI3K-AKT-Rac1 pathway in porcine reproductive and respiratory syndrome virus entry. Biochem Biophys Res Commun 458:392–398
Nishimura R, Tabata K, Arakawa M, Ito Y, Kimura Y, Akihisa T, Nagai H, Sakuma A, Kohno H, Suzuki T (2007) Isobavachalcone, a chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma. Biol Pharm Bull 30:1878–1883
Renson P, Fablet C, Le Dimna M, Mahe S, Touzain F, Blanchard Y, Paboeuf F, Rose N, Bourry O (2017) Preparation for emergence of an Eastern European porcine reproductive and respiratory syndrome virus (PRRSV) strain in Western Europe: immunization with modified live virus vaccines or a field strain confers partial protection. Vet Microbiol 204:133–140
Renukaradhya GJ, Meng XJ, Calvert JG, Roof M, Lager KM (2015) Live porcine reproductive and respiratory syndrome virus vaccines: current status and future direction. Vaccine 33:4069–4080
Shi M, Holmes EC, Brar MS, Leung FC (2013) Recombination is associated with an outbreak of novel highly pathogenic porcine reproductive and respiratory syndrome viruses in China. J Virol 87:10904–10907
Sun N, Li E, Wang Z, Zhao J, Wang S, He J, Bai Y, Li H (2014) Sodium tanshinone IIA sulfonate inhibits porcine reproductive and respiratory syndrome virus via suppressing N gene expression and blocking virus-induced apoptosis. Antiviral Ther 19:89–95
Tang YD, Fang QQ, Liu JT, Wang TY, Wang Y, Tao Y, Liu YG, Cai XH (2016) Open reading frames 1a and 1b of the porcine reproductive and respiratory syndrome virus (PRRSV) collaboratively initiate viral minus-strand RNA synthesis. Biochem Biophys Res Commun 477:927–931
Tang YD, Liu JT, Fang QQ, Wang TY, Sun MX, An TQ, Tian ZJ, Cai XH (2016) Recombinant pseudorabies virus (PRV) expressing firefly luciferase effectively screened for CRISPR/Cas9 single guide RNAs and antiviral compounds. Viruses 8:90
Tian K (2017) NADC30-like porcine reproductive and respiratory syndrome in China. Open Virol J 11:59–65
Tianchao Wei Z, An Tongqing, Zhou Yanjun, YifengJiang Xiaofang Hao, Zhang Shanrui, Peng Jinmei, Qiu Hua-ji, Tong Guang-zhi (2008) Development and application of Taq Man-MGB fluorescence quantitative RT-PCR assay for detection of porcine reproductive and respiratory syndrome virus. Chin J Prev Vet Med 30:6
Wang G, Yu Y, Zhang C, Tu Y, Tong J, Liu Y, Chang Y, Jiang C, Wang S, Zhou EM, Cai X (2016) Immune responses to modified live virus vaccines developed from classical or highly pathogenic PRRSV following challenge with a highly pathogenic PRRSV strain. Dev Comp Immunol 62:1–7
Wang LJ, Xie W, Chen XX, Qiao S, Zhao M, Gu Y, Zhao BL, Zhang G (2017) Molecular epidemiology of porcine reproductive and respiratory syndrome virus in Central China since 2014: the prevalence of NADC30-like PRRSVs. Microb Pathog 109:20–28
Wang R, Wang X, Wu JQ, Ni B, Wen LB, Huang L, Liao Y, Tong GZ, Ding C, Mao X (2016) Efficient porcine reproductive and respiratory syndrome virus entry in MARC-145 cells requires EGFR-PI3K-AKT-LIMK1-COFILIN signaling pathway. Virus Res 225:23–32
Wang X, Zhang H, Abel AM, Young AJ, Xie L, Xie Z (2014) Role of phosphatidylinositol 3-kinase (PI3K) and Akt1 kinase in porcine reproductive and respiratory syndrome virus (PRRSV) replication. Arch Virol 159:2091–2096
Wenhui L, Zhongyan W, Guanqun Z, Zhili L, JingYun M, Qingmei X, Baoli S, Yingzuo B (2012) Complete genome sequence of a novel variant porcine reproductive and respiratory syndrome virus (PRRSV) strain: evidence for recombination between vaccine and wild-type PRRSV strains. J Virol 86:9543
Zhang H, Wang X (2010) A dual effect of porcine reproductive and respiratory syndrome virus replication on the phosphatidylinositol-3-kinase-dependent Akt pathway. Arch Virol 155:571–575
Zhang Q, Jiang P, Song Z, Lv L, Li L, Bai J (2016) Pathogenicity and antigenicity of a novel NADC30-like strain of porcine reproductive and respiratory syndrome virus emerged in China. Vet Microbiol 197:93–101
Zhao C, Liu S, Li C, Yang L, Zu Y (2014) In vitro evaluation of the antiviral activity of the synthetic epigallocatechin gallate analog-epigallocatechin gallate (EGCG) palmitate against porcine reproductive and respiratory syndrome virus. Viruses 6:938–950
Zhao K, Ye C, Chang XB, Jiang CG, Wang SJ, Cai XH, Tong GZ, Tian ZJ, Shi M, An TQ (2015) Importation and recombination are responsible for the latest emergence of highly pathogenic porcine reproductive and respiratory syndrome virus in China. J Virol 89:10712–10716
Zhou L, Yang H (2010) Porcine reproductive and respiratory syndrome in China. Virus Res 154:31–37
Zhou L, Wang Z, Ding Y, Ge X, Guo X, Yang H (2015) NADC30-like strain of porcine reproductive and respiratory syndrome virus, China. Emerg Infect Dis 21:2256–2257
Zhou L, Yang B, Xu L, Jin H, Ge X, Guo X, Han J, Yang H (2017) Efficacy evaluation of three modified-live virus vaccines against a strain of porcine reproductive and respiratory syndrome virus NADC30-like. Vet Microbiol 207:108–116
Acknowledgements
This study was supported by the National Key R&D Program (grant number 2016YFD0500100) and the National Science Foundation of Heilongjiang (grant number ZD2015006).
Funding
This study was funded by the National Key R&D Program (grant number 2016YFD0500100) and the National Science Foundation of Heilongjiang (grant number ZD2015006).
Author information
Authors and Affiliations
Contributions
Y-D Tang and X-H Cai designed the experiments. H-M Wang and other others performed the experiments. All authors analyzed the data. H-M Wang, Y-D Tang and X-H Cai wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The experimental protocols for preparing porcine alveolar macrophages (PAMs) were approved by the Animal Care and Protection Committee of Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences.
Additional information
Handling Editor: Zhongjie Shi.
Rights and permissions
About this article
Cite this article
Wang, HM., Liu, TX., Wang, TY. et al. Isobavachalcone inhibits post-entry stages of the porcine reproductive and respiratory syndrome virus life cycle. Arch Virol 163, 1263–1270 (2018). https://doi.org/10.1007/s00705-018-3755-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-3755-4