Abstract
1-Methylimidazolium trifluoroacetate ([Hmim]TFA) is reported as a cost-effective catalyst for a simple and environmentally benign hetero-Michael reaction. [Hmim]TFA works both as reaction medium and catalyst. The reaction is applicable to various aromatic sulfur and nitrogen nucleophiles. This method has advantages such as high yields, short reaction time, and simple workup. The catalyst could be recycled several times without any loss of activity.
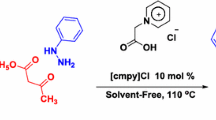
Graphical abstract


Similar content being viewed by others
References
Sharma YO, Degani MS (2007) J Mol Catal A Chem 277:215
Cardillo G, Tomasini C (1996) Chem Soc Rev 25:117
Fustero S, Pina B, Salaver E (2002) J Org Chem 67:4667
Fujita E, Nagao Y (1977) Bioorg Chem 6:287
Sheldon RA (1993) Chirotechnology: industrial synthesis of optically active compounds. Marcel Dekker Publishing, New York
Jenner G (1995) Tetrahedron Lett 36:233
Azizi N, Saidi MR (2004) Tetrahedron 60:383
Duan Z, Xuan X, Li T, Wu Y (2006) Tetrahedron Lett 47:5433
Varala R, Alam MM, Adapa SR (2003) Synlett 720
Xu LW, Li L, Xia CG (2004) Helv Chim Acta 87:1522
Kantam ML, Neeraja V, Kavita B, Neelima B, Chaudhuri MK, Hussain S (2005) Adv Synth Catal 347:763
Chaudhuri MK, Hussain S, Kantam ML, Neelima B (2005) Tetrahedron Lett 46:8329
Surendra K, Krishnaveni NS, Sridhar R, Rao KR (2006) Tetrahedron Lett 47:2125
Hashemi MM, Eftekhari-Sis B, Abdollahifar A, Khalili B (2006) Tetrahedron 62:672
Fetterly BM, Jana NK, Verkade JG (2006) Tetrahedron 62:440
Sakai N, Annaka K, Konakahara T (2006) Tetrahedron Lett 47:631
Ahmed N, Van Lier JE (2006) Tetrahedron Lett 47:2725
Zhang HX, Zhang YH, Liu LF, Xu HL, Wang YG (2005) Synthesis 2129
Karodia N, Liu X, Ludley P, Pletsas D, Stevenson G (2006) Tetrahedron 62:11039
Tommasi I, Sorrentino F (2009) Tetrahedron Lett 50:104
Sotgiu G, Chiarotto I, Feroci M, Orsini M, Rossi I, Inesi A (2008) Electrochem Acta 53:7852
Paape N, Wei W, Bosmann A, Kolbeck C, Maier F, Steinruek HP, Wasserscheid P, Schulz PS (2008) Chem Commun 3867
Ou WH, Huang ZZ (2006) Green Chem 8:731
Ranu BC, Jana R (2005) J Org Chem 70:8621
Driver G, Johnson KE (2003) Green Chem 5:163
Dabiri M, Salehi P, Baghbanzadeh M, Shakouri M, Otokesh S, Ekrami T, Doosti R (2007) J Iran Chem Soc 4:393
Dabiri M, Baghbanzadeh M, Arzroomchilar E (2008) Catal Commun 9:939
Dabiri M, Salehi P, Baghbanzadeh M, Nikcheh MS (2008) Tetrahedron Lett 49:5366
Dabiri M, Baghbanzadeh M, Delbari AS (2008) J Comb Chem 10:700
Dabiri M, Salehi P, Otokesh S, Baghbanzadeh M, Kozehgary G, Mohammadi AA (2005) Tetrahedron Lett 46:6123
Salehi P, Dabiri M, Zolfigol MA, Otokesh S, Baghbanzadeh M (2006) Tetrahedron Lett 47:2557
Dabiri M, Baghbanzadeh M, Nickcheh MS, Arzroomchilar E (2008) Bioorg Med Chem Lett 18:436
Dabiri M, Salehi P, Baghbanzadeh M, Agheb M, Heydari S (2008) Catal Commun 9:785
Bhanushali MJ, Nandurkar NS, Jagtap SR, Bhanage BM (2008) Catal Commun 9:1189
Yang L, Xu LW, Zhou W, Li L, Xia CG (2006) Tetrahedron Lett 47:7723
Liu M, Sibi MP (2002) Tetrahedron 58:7991
Xu JM, Wu Q, Zhang QY, Zhang F, Lin XF (2007) Eur J Org Chem 1798
Sharma G, Kumar R, Chakraborti AK (2007) J Mol Catal A: Chem 263:143
Wang X, Li Z, Zhu X, Maoa H, Zou X, Kong L, Li X (2008) Tetrahedron 64:6510
Bedos P, Amblard M, Subra G, Dodey P, Luccarini JM, Paquet JL, Pruneau D, Aumelas A, Martinez J (2000) J Med Chem 43:2387
Itabashi S, Lu R, Miyakoshi T (2011) Heterocycles 83:171
Weinstein HA, Pierson MR, Wargotz B, Yen FT (1958) J Org Chem 23:363
Acknowledgments
The authors would like to acknowledge financial support from the Research Council of Shahid Beheshti University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dabiri, M., Salehi, P., Bahramnejad, M. et al. Ecofriendly and efficient procedure for hetero-Michael addition reactions with an acidic ionic liquid as catalyst and reaction medium. Monatsh Chem 143, 109–112 (2012). https://doi.org/10.1007/s00706-011-0570-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-011-0570-y