Abstract
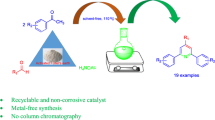
An efficient and entirely green protocol for preparation of 1-(amido/amino)alkyl-2-naphthols via one-pot multi-component reaction of an aldehyde, 2-naphthol or phenol and amides or amines using activated Fuller’s earth as a heterogeneous catalyst under the solvent-free condition is described. This catalyst provides several advantages such as low-cost, stability, reusability, and excellent yields. High catalytic activity and ease of recovery are additional eco-friendly attributes of this catalytic system.
Graphical abstract


Similar content being viewed by others
References
Thomas JM, Thomas WJ (1996) Principles and practice of heterogeneous catalysis. Wiley-VCH, Weinheim
Smith GV, Notheisz F (2000) Heterogeneous catalysis in organic chemistry. Elsevier
Strecker A (1850) Justus Liebigs Ann Chem 75:27
Terrett NK, Gardner M, Gordon DW, Kobylecki RJ, Steele J (1995) Tetrahedron 51:8135
Thompson LA, Ellman JA (1996) Chem Rev 96:555
Dömling A, Ugi I (2000) Angew Chem Int Ed 39:3168
Ahmed N, van Lier JE (2007) Tetrahedron Lett 48:5407
Dömling A (2006) Chem Rev 106:17
Cardellicchio C, Capozzi MA, Naso F (2010) Tetrahedron Asymmetry 21:507
Betti M (1941) In: Gilman H (ed) Organic synthesis collective, vol 1. Wiley, New York, p 381
Knapp S (1995) Chem Rev 95:1859
Seebach D, Matthews JL (1997) Chem Commun 21:2015
Juaristi E (1997) Enantioselective synthesis of β-amino acids. Wiley, New York
Shen AY, Tsai CT, Chen CL (1999) Eur J Med Chem 34:877
Dingermann T, Steinhilber D, Folkes G (2004) Molecular biology in medicinal chemistry. Wiley-VCH
Remillard S, Rebhun LI, Howie GA, Kupchan SM (1975) Science 189:1002
Lesher GY, Surrey AR (1955) J Am Chem Soc 77:636
Mosher HS, Frankel MB, Gregory M (1953) J Am Chem Soc 75:5326
Peglion JL, Vian J, Goument B, Despaux N, Audinot V, Millan MJ (1997) Bioorg Med Chem Lett 7:881
Ren H, Grady S, Gamenara D, Heinzen H, Moyna P, Croft SL, Moyna G (2001) Bioorg Med Chem Lett 11:1851
Clark RD, Caroon JM, Kluge AF, Repke DB, Roszkowski AP, Strosberg AM, Okada MD (1983) J Med Chem 26:657
Matsuoka H, Ohi N, Mihara M, Suzuki H, Miyamoto K, Maruyama N, Yano K (1997) J Med Chem 40:105
Hulst R, Heres H, Peper NC, Kellogg RM (1996) Tetrahedron Asymmetry 7:1373
Li X, Yeung CH, Chan AS, Yang TK (1999) Tetrahedron Asymmetry 10:759
Abou-Elmagd WS, Hashem AI (2013) Med Chem Res 22:2005
Khodaei MM, Khosropour AR, Moghanian H (2006) Synlett 2006:916
Shaterian HR, Yarahmadi H, Ghashang M (2008) Bioorg Med Chem Lett 18:788
Nandi GC, Samai S, Kumar R, Singh MS (2009) Tetrahedron Lett 50:7220
Hajipour AR, Ghayeb Y, Sheikhan N, Ruoho AE (2009) Tetrahedron Lett 50:5649
Safari J, Zarnegar Z (2013) J Mol Catal A: Chem 379:269
Kumar A, Gupta MK, Kumar M (2012) RSC Adv 2:7371
Davoodnia A, Mahjoobin R, Tavakoli-Hoseini N (2014) Chin J Catal 35:490
Hajjami M, Ghorbani F, Bakhti F (2014) Appl Catal A: Gen 470:303
Shaterian HR, Yarahmadi H, Ghashang M (2008) Tetrahedron 64:1263
Supal AR, Gokavi GS (2010) J Chem Sci 122:189
Kantevari S, Vuppalapati SV, Nagarapu L (2007) Catal Commun 8:1857
Shaterian HR, Yarahmadi H (2008) Tetrahedron Lett 49:1297
Das B, Laxminarayana K, Ravikanth B, Rao BR (2007) J Mol Catal A: Chem 261:180
Samantaray S, Hota G, Mishra BG (2011) Catal Commun 12:1255
Nagarapu L, Baseeruddin M, Apuri S, Kantevari S (2007) Catal Commun 8:1729
Kundu D, Majee A, Hajra A (2010) Catal Commun 11:1157
Das VK, Borah M, Thakur AJ (2013) J Org Chem 78:3361
Safari J, Zarnegar Z (2014) J Ind Eng Chem 20:2292
Datta B, Pasha MA (2011) Ultrason Sonochem 18:624
Rekunge DS, Indalkar KS, Chaturbhuj GU (2016) Tetrahedron Lett 57:5815
Khatri CK, Rekunge DS, Chaturbhuj GU (2016) New J Chem 40:10412
Khatri CK, Satalkar VB, Chaturbhuj GU (2017) Tetrahedron Lett 58:694
Rekunge DS, Khatri CK, Chaturbhuj GU (2017) Tetrahedron Lett 58:1240
Indalkar KS, Khatri CK, Chaturbhuj GU (2017) J Chem Sci 129:141
Indalkar KS, Khatri CK, Chaturbhuj GU (2017) J Chem Sci 129:415
Khatri CK, Patil MS, Chaturbhuj GU (2017) J Iran Chem Soc 14:1683
Khatri CK, Mali AS, Chaturbhuj GU (2017) Monatsh Chem 148:1463
Indalkar KS, Khatri CK, Chaturbhuj GU (2017) Tetrahedron Lett 58:2144
Rekunge DS, Khatri CK, Chaturbhuj GU (2017) Monatsh Chem 148:2091
Patil MS, Palav AV, Khatri CK, Chaturbhuj GU (2017) Tetrahedron Lett 58:2859
Patil MS, Mudaliar C, Chaturbhuj GU (2017) Tetrahedron Lett 58:3250
Khatri CK, Chaturbhuj GU (2017) J Iran Chem Soc 14:2513
Indalkar KS, Patil MS, Chaturbhuj GU (2017) Tetrahedron Lett 58:4496
Rekunge DS, Khatri CK, Chaturbhuj GU (2017) Tetrahedron Lett 58:4304
Jejurkar VP, Khatri CK, Chaturbhuj GU, Saha S (2017) Chem Select 2:11693
Patil MS, Khatri CK, Chaturbhuj GU (2018) Monatsh Chem. https://doi.org/10.1007/s00706-018-2169-z
Mali AS, Potnis CS, Chaturbhuj GU (2018) J Iran Chem Soc 15:1399
Bamoniri A, Mirjalili BF, Nazemian S (2014) J Iran Chem Soc 11:653
Hajjami M, Bakhti F, Ghiasbeygi E (2015) Croat Chem Acta 88:197
Kiasat AR, Hemat-Alian L, Saghanezhad SJ (2016) Res Chem Intermed 42:915
Nasresfahani Z, Kassaee MZ, Eidi E (2016) New J Chem 40:4720
Zare A (2012) Org Prep Proced Int 44:82
Zare A, Hasaninejad A, Rostami E, Moosavi-Zare AR, Pishahang N, Roshankar M, Khedri M (2010) J Chem 7:1162
Zali A, Shokrolahi A (2012) Chin Chem Lett 23:269
Moeinpour F, Sardashti-Birjandi A, Dorostkar-Ahmadi N, Khojastehnezhad A, Mohseni-Shahri FS (2012) Synth React Inorg, Met-Org, Nano-Met Chem 42:278
Ghodrati K, Farrokhi A, Karami C, Hamidi Z (2015) Synth React Inorg, Met-Org, Nano-Met Chem 45:15
Gupta A, Kour D, Gupta VK, Kapoor KK (2016) Tetrahedron Lett 57:4869
Ghomi JS, Zahedi S (2013) Monatsh Chem 144:687
Shaterian HR, Hosseinian A, Ghashang M (2008) Synth Commun 38:3375
Srihari G, Nagaraju M, Murthy MM (2007) Helv Chim Acta 90:1497
Wang M, Liang Y, Zhang T, Gao J (2011) Chin J Chem 29:1656
Nasr-Esfahani M, Montazerozohori M, Taei M (2016) C R Chim 19:986
Hakimi F (2016) J Chem Res 40:489
Taghrir H, Ghashang M, Biregan MN (2016) Chin Chem Lett 27:119
Tayebee R, Amini MM, Akbari M, Aliakbari A (2015) Dalton Trans 44:9596
Wang C, Wan Y, Wang HY, Zhao LL, Shi JJ, Zhang XX, Wu H (2013) J Heterocycl Chem 50:496
Maleki B, Taimazi F (2014) Org Prep Proced Int 46:252
Pourmousavi SA, Moghimi P, Ghorbani F, Zamani M (2017) J Mol Struct 1144:87
Azizi N, Edrisi M (2017) Res Chem Intermed 43:379
Acknowledgements
The authors are grateful to University Grants Commission, India for their financial supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rekunge, D.S., Bendale, H.S. & Chaturbhuj, G.U. Activated Fuller’s earth: an efficient, inexpensive, environmentally benign, and reusable catalyst for rapid solvent-free synthesis of 1-(amido/amino)alkyl-2-naphthols. Monatsh Chem 149, 1991–1997 (2018). https://doi.org/10.1007/s00706-018-2247-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2247-2