Abstract
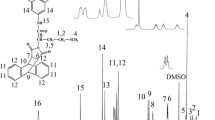
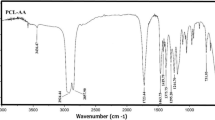
In this investigation, a series of thermally stable and optically active polyamides (PA)s containing bulky pendant chiral functionality from polymerization of a diacid monomer containing rigid phthalimide and flexible l-leucine groups, (2S)-5-[4-(4-methyl-2-phthalimidylpentanoylamino)benzoylamino]isophthalic acid with several aromatic and aliphatic diisocyanates such as 4,4′-methylenebis(phenyl isocyanate), toluylene-2,4-diisocyanate, isophorone diisocyanate, and hexamethylene diisocyanate under gradual heating method were prepared and compared with microwave-assisted polycondensation method. The polymerization reactions occurred rapidly under microwave irradiation and produced a series of PAs with good yields and moderate inherent viscosities of 0.26–0.68 dL/g. All of the new PAs showed good solubility and were readily dissolved in aprotic organic solvents. The resulting polymers were characterized by FT-IR, 1H NMR spectroscopy, and elemental analysis technique. Thermal stability and thermal properties of PAs were evaluated by thermogravimetric analysis and differential scanning calorimetry. The interpretation of kinetic parameters (E, ∆H, ∆S, and ∆G) of thermal decomposition stages have been evaluated using Coats–Redfern equations.





Similar content being viewed by others
References
Bogdal D, Gorczyk J (2004) Synthesis and characterization of epoxy resins prepared under microwave irradiation. J Appl Polym Sci 94:1969–1975
Bogdal D, Loupy A (2008) Application of microwave irradiation to phase-transfer catalyzed reactions. Org Process Res Dev 12:710–722
Brooks GA (1987) Amino acid and protein metabolism during exercise and recovery. Med Sci Sports Exerc 19:S150–S156
Cianga L (2003) Synthesis and characterization of optically active polymers containing azo groups and (L)-α-amino acid moieties. Eur Polym J 39:2271–2282
Diakoumakos CD, Mikroyannidis JA (1994) Polyisophthalimides with pendent phthalimide groups. Polymer 35:1986–1990
Feng L, Hu J, Liu Z, Zhao F, Liu G (2007) Preparation and properties of optically active poly(N-methacryloyl L-leucine methyl ester). Polymer 48:3616–3623
Frazer AH (1968) High temperature resistant polymers. Polymer reviews, vol 17. Interscience, New York
Frexes-Steed M, Lacy DB, Collins J, Abumrad NN (1992) Role of leucine and other amino acids in regulating protein metabolism in vivo. Am J Physiol 262:E925–E935
Garlick PJ (2005) The role of leucine in the regulation of protein metabolism. J Nutr 135:1553S–1556S
Gedye R, Smith F, Westaway K, Humera A, Baldsera L, Laberge L, Rousel L (1986) The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett 27:279–282
Glguere R, Bray Y, Duncan SM, Majetich G (1986) Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett 27:4945–4948
Hoogenboom R, Schubert US (2007) Microwave-assisted polymer synthesis: recent developments in a rapidly expanding field of research. Macromol Rapid Commun 28:368–386
Iwamura T, Ashizawa K, Sakaguchi M (2009) Efficient and eco-friendly anionic polymerization of acrylamide under microwave irradiation and hydrolysis of the obtained polymers by microwave irradiation. Macromolecules 42:5001–5006
Layman DK (2003) The role of leucine in weight loss diets and glucose homeostasis. J Nutr 133:261S–267S
Layman DK, Baum JI (2004) Dietary protein impact on glycemic control during weight loss. J Nutr 134:968S–973S
Li Q, Xu Z, Yi C (2008) Preparation of poly(amic acid) and polyimide derived from 3, 3’, 4, 4’-benzophenonetetracarboxylic dianhydride with different diamines by microwave irradiation. J Appl Polym Sci 107:797–802
Loupy A (ed) (2002) Microwave in organic synthesis. Wilet-VCH Verlag GmbH, Weinheim
Mallakpour S, Rafiee Z (2008a) Application of microwave-assisted reactions in step-growth polymerization: a review. Iran Polym J 17:907–935
Mallakpour S, Rafiee Z (2008b) Microwave-induced synthesis of new optically active and soluble polyamides containing pendent 4-(2-phthalimidiylpropanoyl amino)-benzoylamino-groups. Amino Acids (in press)
Mallakpour S, Rafiee Z (2009) Expeditious synthesis of novel aromatic polyamides from 5-[3-phenyl-2-(9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboximido)- propanoylamino]isophthalic acid and various diamines using microwave-assisted polycondensation. React Funct Polym 69:252–258
Mallakpour S, Sepehri S (2008) Synthesis and characterization of new optically active polyesters by step-growth polymerization of novel aromatic (2S)-4-[(4-methyl-2-phthalimidylpentanoylamino) benzoylamino]isophthalic acid with aromatic diols. J Appl Polym Sci 110:2942–2949
Mallakpour S, Seyedjamali H (2008) Synthesis and characterization of novel organosoluble and optically active aromatic polyesters containing L-methionine and phthalimide pendent groups. Amino Acids 34:531–538
Marcos-Fernandez A, Lozano AE, Abajo JD, De La Campa JG (2001) Novel aromatic polyamides with 1, 3-bezoazole groups in the main chain.1. Polymers derived from 2-(4-carboxyphenyl) benzoxazole-5- and 6-carboxylic acids. Synthesis and characterizations. Polymer 42:7933–7941
Massey KA, Blakeslee CH, Pitkow HS (1998) A review of physiological and metabolic effects of essential amino acids. Amino Acids 14:271–300
Melo-Junior C, Albuquerque C, Fortuny M, Dariva C, Egues S, Santos AF, Ramos A (2009) Use of microwave irradiation in the noncatalytic esterification of C18 fatty acids. Energ Fuel 23:580–585
Mikroyannidis JA (1996) Aromatic polyamides and polyimides with benzimidazole or benzoxazinone pendent groups prepared from 5-(2-benzimidazole)- or 5-(2- benzoxazinone)-1, 3-phenylenediamine. Polymer 37:2715–2721
Nair KS, Schwartz RG, Welle S (1992) Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol 263:E928–E934
Nakano T (2001) Optically active synthetic polymers as chiral stationary phases in HPLC. J Chromatogr A 906:205–225
Norton LE, Layman DK (2006) Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J Nutr 136:533S–537S
Okamoto Y, Yashima E, Hatada K, Mislow K (1984) Chromatographic resolution of perchlorotriphenylamine on (+)-poly(triphenylmethyl methacrylate). J Org Chem 49:557–558
Refat MS, El-Didamony AM, Grabchev I (2007) UV-vis, IR spectra and thermal studies of charge transfer complex formed between poly(amidoamine) dendrimers and iodine. Spectrochim Acta Part A 67:58–65
Rogers ME, Long TE (2003) Synthetic methods in step-growth polymers. Wiley, Hoboken
Samui AB, Ratna D, Chavan JG, Deb PC (2002) Modification of epoxy resin with optically active carboxylic acid. J Appl Polym Sci 86:2523–2529
Shih YF, Chieh YC (2007) Thermal degradation behavior and kinetic analysis of biodegradable polymers using various comparative models, 1 Poly(butylene succinate). Macromol Theory Simul 16:101–110
Van Krevelen DW, Hoftyzer PJ (1976) Properties of polymers, 3rd edn. Elsevier, Amsterdam
Wiesbroc F, Hoogenboom R, Leenen MAM, Meier MAR, Schubert US (2005) Investigation of the living cationic ring-opening polymerization of 2-methyl-, 2-ethyl-, 2-nonyl-, and 2-phenyl-2-oxazoline in a single-mode microwave reactor. Macromolecules 38:5025–5034
Yang HH (1989) Aromatic high-strenght fibers. Wiley-Interscience, New York
Yoneyama H, Tsujimoto A, Goto H (2007) Preparation of optically active pyridine-based conducting polymer films using a liquid crystal electrolyte containing a cholesterol derivative. Macromolecules 40:5279–5283
Acknowledgments
We wish to express our gratitude to the Research Affairs Division, Isfahan University of Technology (IUT), Isfahan for partial financial support. Further financial support from National Elite Foundation (NEF) and Center of Excellency in Sensors and Green Chemistry Research (IUT) is gratefully acknowledged. We also extend our thanks to Mr. Mehdi Taghavi and Mr. Mohammad Dinari for their valuable assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mallakpour, S., Zadehnazari, A. Microwave irradiation as a versatile tool for increasing reaction rates and yields in synthesis of optically active polyamides containing flexible l-leucine amino acid. Amino Acids 38, 1369–1376 (2010). https://doi.org/10.1007/s00726-009-0347-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-009-0347-3