Abstract
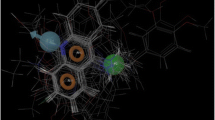
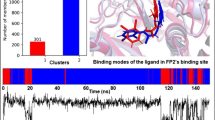
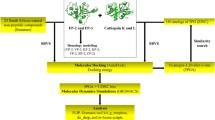
Noncompetitive inhibitors of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) orthologue (PfATP6) of P. falciparum have important therapeutic value in the treatment of malaria. Artemisinin and its analogues are one such class of inhibitors which bind to a hydrophobic pocket located in the transmembrane region of PfATP6 near the biomembrane surface and interfere with calcium transport. The 3D structure of PfATP6 was modeled by homology modeling. A library consisting of 150 artemisinin analogues has been designed. Their molecular interactions and binding affinities with modeled PfATP6 protein have been studied using the docking, molecular mechanics based on generalized Born/surface area (MM-GBSA) solvation model and multi-ligand bimolecular association with energetics (eMBrAcE). Structure activity relationship models were developed between the antimalarial activity (log RA) and molecular descriptors like docking score and binding free energy. For both the cases the r 2 was in the range of 0.538–0.688 indicating good data fit and \( r_{cv}^2 \) was in the range of 0.525–0.679 indicating that the predictive capabilities of the models were acceptable. Besides, a scheme similar to linear response was used to develop free energy of binding (FEB) relationship based on electrostatic (∆G ele), van der Waal (∆G vdW) and surface accessible surface area (SASA), which can express the activity of these artemisinin derivatives. It has been seen that ∆G vdW has most significant correlation to the activity (log RA) and electrostatic energy (∆G ele) has less significant correlation. It indicates that the binding of these artemisinin derivatives to PfATP6 is almost hydrophobic. Low levels of root mean square error for the majority of inhibitors establish the docking, Prime/MM-GBSA and eMBrAcE based prediction model is an efficient tool for generating more potent and specific inhibitors of PfATP6 by testing rationally designed lead compound based on artemisinin derivatization.









Similar content being viewed by others
References
Klayman DL (1985) Qinghaosu (artemisinin) an antimalarial drug from China. Science 228:1049–1055
Arrow KJ, Panosian CB, Geltband H (2004) Saving lives, buying time economics of malaria drugs in an age of resistance. National Academic, Washington, DC
Jefford CW (2001) Why artemisinin and certain synthetic peroxides are potent antimalarials. Implications for the mode of action. Curr Med Chem 8:1803–1826
Pandey AV, Tekwani BL, Singh RL, Chauhan VS (1999) Artemisinin, an endoperoxide antimalarial, disrupts the hemoglobin catabolism and hemin detoxification systems in malarial parasite. J Biol Chem 274:19383–19388
Haynes RK et al (2003) Artemisinin antimalarials do not inhibit hemozoin formation. Antimicrob Agents Chemother 47:1175
O’Neill P et al (2000) Biomimetic Fe(II)-mediated degradation of arteflene (Ro-42–1611). The first EPR spin-trapping evidence for the previously postulated secondary carbon-centered cyclohexyl radical. J Org Chem 65:1578–1582
Hawley SR et al (1998) Relationship between antimalarial drug activity, accumulation, and inhibition of heme polymerization in Plasmodium falciparum in vitro. Antimicrob Agents Chemother 42:682–686
Ellis DS et al (1985) The chemotherapy of rodent malaria, XXXIX. Ultrastructural changes following treatment with artemisinine of Plasmodium berghei infection in mice, with observations of the localization of [3H]-dihydroartemisinine in P. falciparum in vitro. Ann Trop Med Parasitol 79:367–374
ter Kuile F, White NJ, Holloway PH, Pasvol G, Krishna S (1993) Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp Parasitol 76:85–95
Eckstein-Ludwig U, Webb RJ, van Goethem IDA, East JM, Lee AG, Kimura M, O’Neill PM, Bray PG, Ward SA, Krishna S (2003) Artemisinins target the SERCA of Plasmodium falciparum. Nature 424:957–961
Kuhlbrandt W (2004) Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol 5:282–295
Toyoshima C, Nakasako M, Nomura H, Ogawa H (2000) Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 angstrom resolution. Nature 405:647–655
Toyoshima C, Nomura H (2002) Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418:605–611
Toyoshima C, Mizutani T (2004) Crystal structure of the calcium pump with a bound ATP analogue. Nature 430:529–535
Sorensen TLM, Moller JV, Nissen P (2004) Phosphoryl transfer and calcium ion occlusion in the calcium pump. Science 304:1672–1675
Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B (2002) Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498–511
Prime version 1.5, Macromodel version 9.1, Schrodinger, LLC, New York, NY, 2005
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Ramachandran GN, Ramakrishnan C, Sasisekharan V (1963) Stereochemistry of polypeptide chain configurations. J Mol Biol 7:95–99
Eisenberg D, Luthy R, Bowie JU (1997) VERIFY3D: assessment of protein models with three-dimensional profiles. Methods Enzymol 277:396–404
Uhlemann AC, Cameron A, Eckstein-Ludwig U, Fischbarg J, Iserovich P, Zuniga FA, East M, Lee A, Brady L, Haynes RK, Krishna S (2005) Nat Struct Mol Biol 12:628–629
Woolfrey JR, Avery MA, Doweyko AM (1998) Comparison of 3D quantitative structure-activity relationship methods: analysis of the in vitro antimalarial activity of 154 artemisinin analogues by hypothetical active-site lattice and comparative molecular field analysis. J Comput-Aided Mol Des 12(2):165–181
Acton N, Karle JM, Miller RE (1993) Synthesis and antimalarial activity of some 9-substituted artemisinin derivatives. J Med Chem 36:2552–2557
Lin AJ, Margaret L, Klayman DL (1989) Antimalarial activity of new water-soluble dihydroartemisinin derivatives. 2. Stereospecificity of the ether side chain. J Med Chem 32:1249–1252
Posner GH, Oh CH, Gerena L, Milhous WK (1992) Extraordinarily potent antimalarial compounds: new, structurally simple, easily synthesized, tricyclic 1, 2, 4-trioxanes. J Med Chem 35:2459–2467
Avery MA, Bonk JD, Chong WKM, Mehrotra S, Miller R (1995) Structure-activity relationships of the antimalarial agent artemisinin. 2. Effect of heteroatom substitution at O-11: synthesis and bioassay of N-Alkyl-11-aza-9-desmethylartemisinins. J Med Chem 38:5038–5044
Avery MA, Mehrotra S, Bonk JD, Vroman JA, Goins DK (1996) Structure-activity relationships of the antimalarial agent artemisinin 4. Effect of substitution at C-3. J Med Chem 39:2900–2906
Pinheiro JC, Ferreira MMC, Romero OAS (2001) Antimalarial activity of dihydroartemisinin derivatives against P. falciparum resistant to mefloquine: a quantum chemical and multivariate study. J Mol Struct THEOCHEM 572:35–44
Leban I, Golic L, Japelj M (1988) Crystal and molecular structure of qinghaosu: a redetermination. Acta Pharm Jugosl 38:71–77
Lisgarten JN, Potter BS, Bantuzeko C, Palmer RA (1998) Structure, absolute configuration, and conformation of the antimalarial compound, artemisinin. J Chem Crystallogr 28:539–543
Bernardinelli G, Jefford CW, Maric D, Thomson C, Weber J (1994) Computational studies of the structures and properties of potential anti-malarial compounds based on the 1, 2, 4-trioxane ring structure: I artemisinin-like molecules. Int J Quant Chem: Quant Biol Symp 21:117–131
Posner GH, Cumming JN, Ploypradith P, Oh CH (1995) Evidence for Fe(iv) O in the molecular mechanismof action of the trioxane antimalarial artemisinin. J Am Chem Soc 117:5885–5886
Haynes RK, Vonwiller SC (1996) The behaviour of qinghaosu (artemisinin) in the presence of hemin iron (II) and (III). Tetrahedron Lett 37:253–256
Rafiee MA, Hadipour NL, Naderi-manesh H (2005) The role of charge distribution on the antimalarial activity of artemisinin analogs. J Chem Inf Model 45:366–370
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring 1 method and assessment of docking accuracy. J Med Chem 47:1739–1749
Eldridge MD, Murray CW, Auton TR, Paolini GV, Mee RP (1997) Empirical scoring functions: the development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J Comput-Aided Mol Des 11:425–445
Guvench O, Weiser J, Shenkin PS, Kolossváry I, Still WC (2002) Application of the frozen atom approximation to the GB/SA continuum model for solvation free energy. J Comput Chem 23:214–221
Wu X, Milne JLS, Borgnia MJ, Rostapshov AV, Subramaniam S, Brooks BR (2003) A core-weighted fitting method for docking atomic structures into low-resolution maps: application to cryo-electron microscopy. J Struct Biol 141:63–76
Todorov NP, Mancera RL, Monthoux PH (2003) A new quantum stochastic tunnelling optimisation method for protein-ligand docking. Chem Phys Lett 369:257–263
Reynolds CH (1995) Estimating liphophilicity using GB/SA continuum solvation Model: a direct method for computing partition coefficients. J Chem Inf Comput Sci 35:738–742
Haynes RK, Krishna S (2004) Artemisinins: activities and actions. Microbes Infect 6:1339–1346
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naik, P.K., Srivastava, M., Bajaj, P. et al. The binding modes and binding affinities of artemisinin derivatives with Plasmodium falciparum Ca2+-ATPase (PfATP6). J Mol Model 17, 333–357 (2011). https://doi.org/10.1007/s00894-010-0726-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0726-4