Abstract
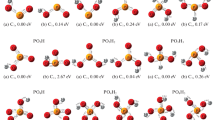
Quantum chemical calculations were used to analyze the chemical bonding and the reactivity of phosphorus oxides (P4O6+n (n = 0–4)). The chemical bonding was studied using topological analysis such as atoms in molecules (AIM), electron localization function (ELF), and the reactivity using the Fukui function. A classification of the P-O bonds formed in all structures was done according to the coordination number in each P and O atoms. It was found that there are five P-O bond types and these are distributed among the five phosphorus oxides structures. Results showed that there is good agreement among the evaluated properties (length, bond order, density at the critical point, and disynaptic population) and each P-O bond type. It was found that regardless of the structure in which a P-O bond type is present the topological and geometric properties do not have a significant variation. The topological parameters electron density and Laplacian of electron density show excellent linear correlation with the average length of P-O bond in each bond type for each structure. From the Fukui function analysis it was possible to predict that from P4O6 until P4O8 the most reactive regions are basins over the P.






Similar content being viewed by others
References
Salvadó MA, Pertierra P (2008) Theoretical study of P2O5 polymorphs at high pressure: hexacoordinated phosphorus. Inorg Chem 47(11):4884–4890
Engels B, Soares Valentim AR, Peyerimhoff SD (2001) About the chemistry of phosphorus suboxides. Angew Chem Int Ed 40(2):378–381
Dimitrov A, Ziemer B, Hunnius W-D, Meisel M (2003) The first ozonide of a phosphorus oxide—preparation, characterization, and structure of P4O18. Angew Chem Int Ed 42(22):2484–2486
Klapötke TM (2003) P4O18—the first binary phosphorus oxide ozonide. Angew Chem Int Ed 42(30):3461–3462
Carbonnière P, Pouchan C (2008) Vibrational spectra for P4O6 and P4O10 systems: theoretical study from DFT quartic potential and mixed perturbation-variation method. Chem Phys Lett 462(4–6):169–172
Mielke Z, Andrews L (1989) Infrared spectra of phosphorus oxides (P4O6, P4O7, P4O8, P4O9 and P4O10) in solid argon. J Phys Chem 93(8):2971–2976
Jansen M, Moebs M (1984) Structural investigations on solid tetraphosphorus hexaoxide. Inorg Chem 23(26):4486–4488
Beattie IR, Ogden JS, Price DD (1978) The characterization of molecular vanadium oxide (V4O10), an analog of phosphorus oxide (P4O10). Inorg Chem 17(11):3296–3297
Sharma BD (1987) Phosphorus(V) oxides. Inorg Chem 26(3):454–455
Valentim ARS, Engels B, Peyerimhoff SD, Clade J, Jansen M (1998) A comparative study of the bonding character in the P4On (n = 6–10) series by means of a vibrational analysis. J Phys Chem A 102(21):3690–3696
Mowrey RC, Williams BA, Douglass CH (1997) Vibrational analysis of P4O6 and P4O10. J Phys Chem A 101(32):5748–5752
Lohr LL (1990) An ab initio characterization of the gaseous diphosphorus oxides P2Ox (x = 1–5). J Phys Chem 94(5):1807–1811
Moussaoui Y, Ouamerali O, De Maré GR (2003) Properties of the phosphorus oxide radical, PO, its cation and anion in their ground electronic states: comparison of theoretical and experimental data. Int Rev Phys Chem 22(4):641–675
Butler JE, Kawaguchi K, Hirota E (1983) Infrared diode laser spectroscopy of the PO radical. J Mol Spectrosc 101(1):161–166
Kanata H, Yamamoto S, Saito S (1988) The dipole moment of the PO radical determined by microwave spectroscopy. J Mol Spectrosc 131(1):89–95
Dyke JM, Morris A, Ridha A (1982) Study of the ground state of PO + using photoelectron spectroscopy. J Chem Soc, Faraday Trans 78(12):2077–2082
Zittel PF, Lineberger WC (1976) Laser photoelectron spectrometry of PO-, PH-, and PH2 -. J Chem Phys 65(4):1236–1243
Noury S, Krokidis X, Fuster F, Silvi B (1997) TopMod Package
Flkiger P, Lthi HP, Portmann S, Weber J (2008) MOLEKEL 5.3. Molekel homepage. http://www.cscs.ch/molekel (accessed 20 April 2010)
Bader R (1990) Atoms in molecules. Oxford University Press, New York, A Quantum Theory
Popelier PLA (1996) MORPHY, a program for an automated "atoms in molecules" analysis. Comput Phys Commun 93:212–240
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1873
Chermette H (1999) Chemical reactivity indexes in density functional theory. J Comput Chem 20:129–154
Ayers PW, Anderson JSM, Bartolotti LJ (2005) Perturbative perspectives on the chemical reaction prediction problem. Int J Quantum Chem 101:520–534
Gazquez J (2008) Perspectives on density functional theory Of chemical reactivity. J Mex Chem Soc 52(1):3–10
Yang WT, Parr RG, Pucci R (1984) Electron density, Kohn-Sham frontier orbitals, and Fukui functions. J Chem Phys 81:2862–2863
Ayers PW, Levy M (2000) Perspective on "Density functional approach to the frontier-electron theory of chemical reactivity" by Parr RG, Yang W (1984). Theor Chem Acc 103:353–360
Perdew JP, Parr RG, Levy M, Balduz JL Jr (1982) Density-functional theory for fractional particle number: derivative discontinuities of the energy. Phys Rev Lett 49:1691–1694
Yang WT, Zhang YK, Ayers PW (2000) Degenerate ground states and fractional number of electrons in density and reduced density matrix functional theory. Phys Rev Lett 84:5172–5175
Ayers PW, Parr RG (2000) Variational principles for describing chemical reactions: the Fukui function and chemical hardness revisited. J Am Chem Soc 122:2010–2018
Ayers PW (2008) The continuity of the energy and other molecular properties with respect to the number of electrons. J Math Chem 43(1):285–303
Parr RG, Yang W (1984) Density functional approach to the frontier-electron theory of chemical reactivity. J Am Chem Soc 106(14):4049–4050
Fuentealba P, Chamorro E, Cardenas C (2007) Further exploration of the Fukui function, hardness, and other reactivity indices and its relationships within the Kohn-Sham scheme. Int J Quantum Chem 107:37–45
Ayers PW (2006) Can one oxidize an atom by reducing the molecule that contains It? Phys Chem Chem Phys 8:3387–3390
Bartolotti LJ, Ayers PW (2005) An example where orbital relaxation is an important contribution to the Fukui function. J Phys Chem A 109:1146–1151
Melin J, Ayers PW, Ortiz JV (2007) Removing electrons can increase the electron density: a computational study of negative Fukui functions. J Phys Chem A 111:10017–10019
Cardenas C, Ayers PW, Cedillo A (2011) Reactivity indicators for degenerate states in the density-functional theoretic chemical reactivity theory. J Chem Phys 134(17):174103–174113
Flores-Moreno R (2009) Symmetry conservation in Fukui functions. J Chem Theory Comput 6(1):48–54
Martínez J (2009) Local reactivity descriptors from degenerate frontier molecular orbitals. Chem Phys Lett 478(4–6):310–322
Tiznado W, Chamorro E, Contreras R, Fuentealba P (2005) Comparison among four different ways to condense the Fukui function. J Phys Chem A 109(14):3220–3224
Fuentealba P, Florez E, Tiznado W (2010) Topological analysis of the Fukui function. J Chem Theory Comput 6(5):1470–1478
Osorio E, Ferraro MB, Oña OB, Cardenas C, Fuentealba P, Tiznado W (2011) Assembling small silicon clusters using criteria of maximum matching of the Fukui functions. J Chem Theory Comput 7(12):3995–4001
Florez E, Tiznado W, Mondragón F, Fuentealba P (2005) Theoretical study of the interaction of molecular oxygen with copper clusters. J Phys Chem A 109(34):7815–7821
Tiznado W, Ona OB, Bazterra VE, Caputo MC, Facelli JC, Ferraro MB, Fuentealba P (2005) Theoretical study of the adsorption of H on Sin clusters, (n = 3–10). J Chem Phys 123(21):214302
Tiznado W, Oña OB, Caputo MC, Ferraro MB, Fuentealba P (2009) Theoretical study of the structure and electronic properties of Si3On − and Si6On − (n = 1–6) clusters. Fragmentation and formation patterns. J Chem Theory Comput 5(9):2265–2273
Kohout M (2011) DGrid 4.6. Radebeul
Popelier PLA (2000) Atoms in molecules. An introduction. Pearson Education, Harlow
Acknowledgment
The authors are grateful to EPM (Empresas Pública de Medellín)/CIIEN (Centro de Investigación e Innovación en Energía) and COLCIENCIAS (Departamento Administrativo de Ciencia, Tecnología e innovación) for financing the project 1115-4547-21979, and to the University of Antioquia for the financial support of the “Programa Sostenibilidad 2013-2014”. WT thanks Fondecyt financial support (Grant No 11090431). NA thanks “COLCIENCIAS” and the University of Antioquia for the PhD scholarship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 188 kb)
Rights and permissions
About this article
Cite this article
Acelas, N.Y., López, D., Mondragón, F. et al. Topological analysis of tetraphosphorus oxides (P4O6+n (n = 0–4)). J Mol Model 19, 2057–2067 (2013). https://doi.org/10.1007/s00894-012-1633-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1633-7