Abstract
Schiff bases have many chemical and biological applications in medicine and pharmaceuticals due to the presence of an imine group (−C=N−). These bases are used in many different fields of technology, and in photochemistry because of their photochromic properties. Here, the structural and electronic properties of the Schiff base formed by tacrine and saccharin (TacSac) were explored using density functional theory with the B3LYP, M06-2X, M06L, and ωB97XD functionals in combination with the 6-311++G(d,p) basis set. The time-dependent formalism was used at the B3LYP/6-311++G(d,p) level to obtain electronic transitions. The calculations were repeated in an implicit solvent model mimicking water, using the polarizable continuum model in conjunction with a solvation model based on a density approach. The results indicate that TacSac cannot form spontaneously, but can be obtained in mild reactions. However, the resulting Schiff base displays different characteristics to its monomers. It also has the potential for use in photochemical intramolecular charge-transfer systems.

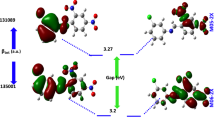
Intramolecular charge transfer between HOMO and LUMO of TacSac









Similar content being viewed by others
References
Freeman SE, Dawson RM (1991) Tacrine: a pharmacological review. Prog Neurobiol 36:257–277. doi:10.1016/0301-0082(91)90002-I
Tumiatti V, Minarini A, Bolognesi ML, Milelli A, Rosini M, Melchiorre C (2010) Tacrine derivatives and Alzheimer’s disease. Curr Med Chem 17:1825–1838. doi:10.2174/092986710791111206
Albert A (1949) The chemical and biological properties of acridines. Sci Prog 37:418–434
Shaw FH, Bentley GA (1953) The pharmacology of some new anticholinesterases. Aust J Exp Biol Med Sci 31:573–576
Kaul PN (1962) Enzyme inhibiting action of tetrahydroaminoacridine and its structural fragments. J Pharm Pharmacol 14:243–248. doi:10.1111/j.2042-7158
Summers WK, Majovski LV, Marsh GM, Tachiki K, Kling A (1986) Oral tetrahydroaminoacridine in long-term treatment of senile dementia, Alzheimer type. N Engl J Med 315:1241–1245. doi:10.1056/NEJM198611133152001
Summers WK (2006) Tacrine, and Alzheimer's treatments. J Alzheimers Dis 9:439–445
Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, Axelsen PH, Silman I, Sussman JL (1993) Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc Natl Acad Sci USA 90:9031–9035
Pishkar L, Jamaat PR, Makarem S (2015) Theoretical study of structure spectral properties of tacrine as Alzheimer drug. J Phys Theor Chem IAU Iran 12(1):69–75
Wong KY, Mercader AG, Saavedra LM, Honarparvar B, Romanelli GP, Duchowicz PR (2014) QSAR analysis on tacrine-related acetylcholinesterase inhibitors. J Biomed Sci 21:84. doi:10.1186/s12929-014-0084-0
Nascimento ÉCM, Martins JBL, dos Santos ML, Gargano R (2008) Theoretical study of classical acetylcholinesterase inhibitors. Chem Phys Lett 458:285–289. doi:10.1016/j.cplett.2008.05.006
Matos MAR, Miranda MS, Morais VMF, Liebman JF (2005) Saccharin: a combined experimental and computational thermochemical investigation of a sweetener and sulfonamide. Mol Phys 103(2–3):221–228. doi:10.1080/00268970512331316175
Kant R (2004) Sweet proteins—potential replacement for artificial low calorie sweeteners. Nutr J 4:5. doi:10.1186/1475-2891-4-5
Weihrauch MR, Diehl V, Bohlen H (2002) Artificial sweeteners—are they potentially carcinogenic? Med Klin (Munich) 96:670–675. doi:10.1007/s000630200011
Kanarek RB (1994) Does sucrose or aspartame cause hyperactivity in children? Nutr Rev 52:173–175. doi:10.1111/j.1753-4887.1994.tb01415.x
Cohen S (2001) What's the truth about the health risks of sugar substitutes such as saccharin and aspartame? Health News 7:10
Nabors LO (1988) Saccharin and aspartame: are they safe to consume during pregnancy? J Reprod Med 33:102
Hagiwara A, Fukushima S, Kitaori M, Shibata M, Ito N (1984) Effects of three sweeteners on rat urinary bladder carcinogenesis initiated by N-butyl-N-(4-hydroxybutyl)-nitrosamine. Gann 75:763–768
Brizuela A, Romano E, Yurquina A, Locatelli S, Brandan SA (2012) Experimental and theoretical vibrational investigation on the saccharinate ion in aqueous solution. Spectrochim Acta A 95:399–406. doi:10.1016/j.saa.2012.04.003
Filimonov VD, Krasnokutskaya EA, Poleshchuk OK, Lesina Yu A, Chaikovskii VK (2006) Electronic structures and reactivities of iodinating agents in the gas phase and in solutions: a density functional study. Russ Chem B+ 55(8):1328–1336. doi:10.1007/s11172-006-0422-9
Branda MM, Castellani NJ, Tarulli SH, Quinzani OV, Baran EJ, Contreras RH (2002) DFT study of electronic structure of saccharin, thiosaccharin, and their respective ıons: effects of metal coordination on thiosaccharinate electronic structure. Int J Quantum Chem 89:525–534. doi:10.1002/qua.10308
Remko M (2003) Theoretical study of molecular structure and gas-phase acidity of some biologically active sulfonamides. J Phys Chem A 107:720–725. doi:10.1021/jp026980m
Jia Y, Li J (2015) Molecular assembly of Schiff base ınteractions: construction and application. Chem Rev 115:1597–1621. doi:10.1021/cr400559g
Xin Y, Yuan J (2012) Schiff's base as a stimuli-responsive linker in polymer chemistry. J Polym Chem 3:3045–3055. doi:10.1039/C2PY20290E
Schiff H (1864) Mittheilungen aus dem Universitätslaboratorium in Pisa: Eine neue Reihe organischer Basen. Justus Liebigs Ann Chem 131:118–119. doi:10.1002/jlac.18641310113
Kratz F, Beyer U, Schutte MT (1999) Drug–polymer conjugates containing acid-cleavable bonds. Crit Rev Ther Drug Carrier Syst 16(3):245–288. doi:10.1615/CritRevTherDrugCarrierSyst.v16.i3.10
Saito H, Hoffman AS, Ogawa HI (2007) Delivery of doxorubicin from biodegradable PEG hydrogels having Schiff base linkages. J Bioact Compat Polym 22:589–601. doi:10.1177/0883911507084653
Chatterjee S, Bhattacharyya S (2015) Schiff bases as a source of potent molecules with anti-cancer potential: a short review. Asian J Biochem Pharm Res 5:86–97
Meenachi S, Chitra S (2014) A review of chemistry and biological importance of Schiff base. Int J Sci Res Rev 3(1):8–18
Rudrapal M, De B (2013) Chemistry and biological importance of heterocyclic Schiff’s bases. Int Res J Pure Appl Chem 3(3):232–249. doi:10.9734/IRJPAC/2013/3996
Al Zoubi W (2013) Biological activities of Schiff bases and their complexes: a review of recent works. Int J Org Chem 3:73–95. doi:10.4236/ijoc.2013.33A008
Kajal A, Bala S, Kamboj S, Sharma N, Saini V (2013) Schiff bases: a versatile pharmacophore. J Catal Article ID 893512:1–14. doi:10.1155/2013/893512
Sathe BS, Jaychandran E, Jagtap VA, Sreenivasa GM (2011) Synthesis, characterization and anti-inflammatory evaluation of new fluorobenzothiazole Schiff’s bases. Int J Pharm Res Dev 3(3):164–169
Sondhi SM, Singh N, Kumar A, Lozach O, Meijer L (2006) Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff’s bases. Bioorg Med Chem 14(11):3758–3765. doi:10.1016/j.bmc.2006.01.054
Pandey A, Dewangan D, Verma S, Mishra A, Dubey RD (2011) Synthesis of Schiff bases of 2-amino-5-aryl-1,3,4-thiadiazole and its analgesic, anti-inflammatory, anti-bacterial and antitubercular activity. Int J ChemTech Res 3(1):178–184
Chandramouli C, Shivanand MR, Nayanbhai TB, Bheemachari B, Udupi RH (2012) Synthesis and biological screening of certain new triazole Schiff bases and their derivatives bearing substituted benzothiazole moiety. J Chem Pharm Res 4(2):1151–1159
Chinnasamy RP, Sundararajan R, Govindaraj S (2010) Synthesis, characterization, and analgesic activity of novel Schiff base of isatin derivatives. J Adv Pharm Technol Res 1(3):342–347. doi:10.4103/0110-5558.72428
Mounika K, Anupama B, Pragathi J, Gyanakumari C (2010) Synthesis, characterization and biological activity of a Schiff base derived from 3-ethoxy salicylaldehyde and 2-amino benzoic acid and its transition metal complexes. J Sci Res 2(3):513–524. doi:10.3329/jsr.v2i3.4899
Venkatesh P (2011) Synthesis, characterization and antimicrobial activity of various Schiff bases complexes of Zn(II) and Cu(II) ions. Asian J Pharm Health Sci 1(1):8–11
Chaubey AK, Pandeya SN (2012) Synthesis & anticonvulsant activity (chemo shock) of Schiff and Mannich bases of isatin derivatives with 2-amino pyridine (mechanism of action). Int J PharmTech Res 4(1):590–598
Aboul-Fadl T, Mohammed FA, Hassan EA (2003) Synthesis, antitubercular activity and pharmacokinetic studies of some Schiff bases derived from 1-alkylisatin and isonicotinic acid hydrazide (INH). Arch Pharm Res 26(10):778–784. doi:10.1007/BF02980020
Miri R, Razzaghi-asl N, Mohammadi MK (2013) QM study and conformational analysis of an isatin Schiff base as a potential cytotoxic agent. J Mol Model 19(2):727–735. doi:10.1007/s00894-012-1586-x
Ali SMM, Abul Kalam Azad M, Jesmin M, Ahsan S, Rahman MM, Khanam JA, Islam MN, Shahriar SMS (2012) In vivo anticancer activity of vanillin semicarbazone. Asian Pac J Trop Biomed 2(6):438–442. doi:10.1016/S2221-1691(12)60072-0
Wei D, Li N, Lu G, Yao K (2006) Synthesis, catalytic and biological activity of novel dinuclear copper complex with Schiff base. Sci China B 49(3):225–229. doi:10.1007/s11426-006-0225-8
Avaji PG, Vinod Kumar CH, Patil SA, Shivananda KN, Nagaraju C (2009) Synthesis, spectral characterization, in-vitro microbiological evaluation and cytotoxic activities of novel macrocyclic bishydrazone. Eur J Med Chem 44(9):3552–3559. doi:10.1016/j.ejmech.2009.03.032
Venugopala KN, Jayashree BS (2003) Synthesis of carboxamides of 2′-amino-4′-(6-bromo-3-coumarinyl) thiazole as analgesic and antiinflammatory agents. Indian J Heterocycl Chem 12(4):307–310
Vashi K, Naik HB (2004) Synthesis of novel Schiff base and azetidinone derivatives and their antibacterial activity. Eur J Chem 1:272–276. doi:10.1155/2004/158924
Brodowska K, Lodyga-Chruscinska E (2014) Schiff bases—interesting range of applications in various fields of science. Chemik 68(2):129–134
Tanaka K, Shimoura R, Caira MR (2010) Synthesis, crystal structures and photochromic properties of novel chiral Schiff base macrocycles. Tetrahedron Lett 51(2):449–452. doi:10.1016/j.tetlet.2009.11.062
Pistolis G, Gegiou D, Hadjoudis E (1996) Effect of cyclodextrin complexation on thermochromic Schiff bases. J Photochem Photobiol A Chem 93(2–3):179–184. doi:10.1016/1010-6030(95)04182-6
Mocanu AS, Ilis M, Dumitrascu F, Ilie M, Circu V (2010) Synthesis, mesomorphism and luminescence properties of palladium(II) and platinum(II) complexes with dimeric Schiff base liquid crystals. Inorg Chim Acta 363(4):729–736. doi:10.1016/j.ica.2009.11.028
Issa YM, Sherif OE, Abbas SM (1998) Chelation behaviour of Ce(III), Th(IV), and UO2(VI) with 5,7-dihydroxy-6-formyl-2-methylbenzopyran-4-one Schiff bases. Monatsh Chem 129:985–998. doi:10.1007/PL00010120
Atta AM, Shaker NO, Maysour NE (2006) Influence of the molecular structure on the chemical resistivity and thermal stability of cured Schiff base epoxy resins. Prog Org Coat 56:100–110. doi:10.1016/j.porgcoat.2005.12.004
Jia JH, Tao XM, Li YJ, Sheng WJ (2011) Synthesis and third-order optical nonlinearities of ferrocenyl Schiff base. Chem Phys Lett 514(1–3):114–118. doi:10.1016/j.cplett.2011.08.035
Ibrahim AMA (1992) Electrical and thermal behaviour of some Schiff bases and their charge transfer complexes with acidic acceptors. Thermochim Acta 197(1):211–217. doi:10.1016/0040-6031(92)87052-C
Kumar S, Dhar DN, Saxena PN (2009) Applications of metal complexes of Schiff bases—a review. J Sci Ind Res 68:181–187
Emregül KC, Düzgün E, Atakol O (2006) The application of some polydentate Schiff base compounds containing aminic nitrogens as corrosion inhibitors for mild steel in acidic media. Corr Sci 48:3243–3260. doi:10.1016/j.corsci.2005.11.016
Ashraf M, Wajid A, Mahmood K, Maah M, Yusoff I (2011) Spectral ınvestigation of the activities of amino substituted bases. Orient J Chem 27(2):363–372
Odén S, von Hofsten B (1954) Detection of fingerprints by the ninhydrin reaction. Nature 173(4401):449–450. doi:10.1038/173449a0
Raczyńska ED, Makowski M, Hallmann M, Kamińska B (2015) Geometric and energetic consequences of prototropy for adenine and its structural models—a review. RSC Adv 5:36587–36604. doi:10.1039/c4ra17280a
Lobanov PS, Dar'in DV (2013) Acetamidines and acetamidoximes containing an electron-withdrawing group at the α-carbon atom: their use in the synthesis of nitrogen heterocycles. Chem Heterocycl Compd 49(4):507–528. doi:10.1007/s10593-013-1277-2
Nowicki J, Muszyński M, Mikkola J-P (2016) Ionic liquids derived from organosuperbases: en route to superionic liquids. RSC Adv 6:9194–9208. doi:10.1039/c5ra23616a
Soeiro MNC, Werbovetz K, Boykin DW, Wilson WD, Wang MZ, Hemphill A (2013) Novel amidines and analogues as promising agents against intracellular parasites: a systematic review. Parasitology 140:929–951. doi:10.1017/S0031182013000292
Michelin RA, Sgarbossa P, Mazzega Sbovata S, Gandin V, Marzano C, Bertani R (2011) Chemistry and biological activity of platinum amidine complexes. ChemMedChem 6:1172–1183. doi:10.1002/cmdc.201100150
Quek JY, Davis TP, Lowe AB (2013) Amidine functionality as a stimulus-responsive building block. Chem Soc Rev 42:7326–7334. doi:10.1039/c3cs60065c
Taylor JE, Bull SD, Williams JMJ (2012) Amidines, isothioureas, and guanidines as nucleophilic catalysts. Chem Soc Rev 41:2109–2121. doi:10.1039/c2cs15288f
Liebeschuetz JW, Katz RB, Duriatti AD, Arnold ML (1997) Rationally designed guanidine and amidine fungicides. Pestic Sci 50:258–274. doi:10.1002/(SICI)1096-9063(199707)50:3<258::AID-PS587>3.0.CO;2-3
Wulff G, Schönfeld R (1998) Polymerizable amidines—adhesion mediators and binding sites for molecular imprinting. Adv Mater 10(12):957–959. doi:10.1002/(SICI)1521-4095(199808)10:12<957::AID-ADMA957>3.0.CO;2-4
Aly AA, Nour-El-Din AM (2008) Functionality of amidines and amidrazones. ARKIVOC i:153–194. doi:10.3998/ark.5550190.0009.106
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision C.01. Gaussian, Inc., Wallingford
Dennington R, Keith T, Millam J (2009) Gaussview 5.0. Semichem Inc., Shawnee Mission
Wavefunction, Inc. (2008) Spartan08 for Windows. Wavefunction, Inc., Irvine
Kohn W, Sham LJ (1965) Self-consistant equations including exchange and correlation effects. Phys Rev 140:A1133–A1138. doi:10.1103/PhysRev.140.A1133
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behaviour. Phys Rev A 38:3098–3100. doi:10.1103/PhysRevA.38.3098
Becke AD (1993) Density-functional thermochemistry III. The role of exact exchange. J Chem Phys 98:5648–5652. doi:10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241. doi:10.1007/s00214-007-0310-x
Zhao Y, Truhlar DG (2006) A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J Chem Phys 125(194101):1–18. doi:10.1063/1.2370993
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620. doi:10.1039/B810189B
Yanai T, Tew D, Handy N (2004) A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57. doi:10.1016/j.cplett.2004.06.011
Cioslowski J (1989) A new population analysis based on atomic polar tensors. J Am Chem Soc 111(22):8333–8336. doi:10.1021/ja00204a001
Tomasi J, Mennucci B, Cancès E (1999) The IEF version of the PCM solvation method: an overview of a new method addressed to study molecular solutes at the QM ab initio level. J Mol Struct THEOCHEM 464:211–226. doi:10.1016/S0166-1280(98)00553-3
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3093. doi:10.1021/cr9904009
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396. doi:10.1021/jp810292n
Acknowledgements
The use of TUBITAK-ULAKBIM TRUBA resources for some of the calculations is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 984 kb)
Rights and permissions
About this article
Cite this article
Acar, N., Selçuki, C. & Coşkun, E. DFT and TDDFT investigation of the Schiff base formed by tacrine and saccharin. J Mol Model 23, 17 (2017). https://doi.org/10.1007/s00894-016-3195-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3195-6