Abstract
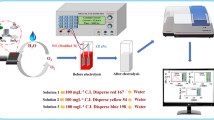
Ozone (O3) has been generated on Ni–Sb–SnO2/Ti electrode as anode immersed in acidic media at 25 °C by electrochemical process. The anode was electrochemically characterized by cyclic voltammetry and morphologically characterized by scanning electron microscopy (SEM) and X-ray diffraction. The concentration of dissolved ozone was determined by a UV/Vis spectrophotometer. The type of electrode with different times coating on the titanium mesh and different acid type and various concentrations (C acid) were used, and the stability of the electrode was investigated under the experimental conditions by SEM images. Results shows that higher efficiency (53.7%) for O3 generation by electrochemical oxidation of water were obtained in HClO4 (1 M) and an applied potential of 2.4 V vs. Ag/AgCl in 150 ml volume undivided electrochemical cell.













Similar content being viewed by others
References
Arihara K, Terashima C, Fujishima A (2007) J Electrochem Soc 154:E71–E75
Pichet P, Hurtubise C (1975) Proceedings of second international symposium on ozone technology, Montreal, Canada, 11–14 May, 1975
Meng MX, Hsieth JJ (2000) Tappi J 83:67–72
Da Silva LM, De Faria LA, Boodts JFC (2003) Electrochim Acta 48:699–709
Stucki S, Theis G, Kötz R, Devantay H, Christen H (1985) J Electrochem Soc 132:367–371
Awad MI, Sata S, Kaneda K, Ikematsu M, Okajima T, Ohsaka T (2006) Electrochem Commun 8:1263–1269
Santana MHP, De Faria LA, Boodts JFC (2004) Electrochim Acta 49:1925–1935
Foller PC, Tobias W (1982) J Electrochem Soc 129:506–515
Chernik AA, Drozdovich VB, Zharskii IM (1997) Russ J Electrochem 33:259–264
Wang YH, Cheng S, Chan KY, Li XY (2005) J Electrochem Soc 152:D197–D200
Da Silva LM, De Faria LA, Boodts JFC (2001) Pure Appl Chem 73:1871–1884
Tatapudi P, Fenton JW (1993) J Electrochem Soc 140:3527–3530
Foller PC, Tobias W (1981) J Phys Chem 85:3238–3244
Chen QY, Shi DD, Zhang YJ, Wang YH (2010) Water Sci Technol 62:2090–2095
Lipp L, Pletcher D (1997) Electrochim Acta 42:1091–1099
Christensen PA, Lin WF, Christensen H, Imkum A, Jin JM, Li G, Dyson CM (2009) Ozone Sci Eng 31:287–293
Bader H, Hoigne J (1981) Water Res 15:449–456
Awad MI, Saleh MM (2010) J Solid State Electrochem 14:1877–1883
Cheng SA, Chan KY (2004) Electrochem Solid State Lett 7:D4–D6
Fernando JB (2004) Ozone reaction kinetics for water and wastewater systems. Lewis, Boca Raton
Jolivet JP (2000) Metal oxide chemistry and synthesis: from solution to oxide. Wiley, New York
He D, Mho S (2004) J Electroanal Chem 568:19–27
Michaud PA, Mahe E, Haenni W, Perret A, Comninellis Ch (2000) Electrochem Solid State Lett 3:77–79
Michaud PA, Panniza M, Outattara L, Daiaco T, Foti G, Comninellis Ch (2003) J Appl Electrochem 33:151–154
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Basiriparsa, J., Abbasi, M. High-efficiency ozone generation via electrochemical oxidation of water using Ti anode coated with Ni–Sb–SnO2 . J Solid State Electrochem 16, 1011–1018 (2012). https://doi.org/10.1007/s10008-011-1440-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-011-1440-6