Abstract
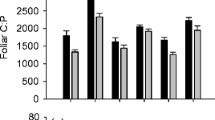
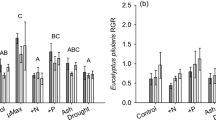
Northern peatlands are recognized as globally important stores of terrestrial carbon (C), yet we have limited understanding of how global changes, including land use, affect C cycling processes in these ecosystems. Making use of a long-term (>50 year old) peatland land management experiment in the UK, we investigated, using a 13CO2 pulse chase approach, how managed burning and grazing influenced the short-term uptake and cycling of C through the plant–soil system. We found that burning affected the composition and growth stage of the plant community, by substantially reducing the abundance of mature ericoid dwarf-shrubs. Burning also affected the structure of the soil microbial community, measured using phospholipid fatty acid analysis, by reducing fungal biomass. There was no difference in net ecosystem exchange of CO2, but burning was associated with an increase in photosynthetic uptake of 13CO2 and increased transfer of 13C to the soil microbial community relative to unburned areas. In contrast, grazing had no detectable effects on any measured C cycling process. Our study provides new insight into how changes in vegetation and soil microbial communities arising from managed burning affect peatland C cycling processes, by enhancing the uptake of photosynthetic C and the transfer of C belowground, whilst maintaining net ecosystem exchange of CO2 at pre-burn levels.






Similar content being viewed by others
References
Aerts R, Verhoeven JTA, Whigham DF. 1999. Plant-mediated controls on nutrient cycling in temperate fens and bogs. Ecology 80:2170–81.
Amiro BD, Ian MacPherson J, Desjardins RL, Chen JM, Liu J. 2003. Post-fire carbon dioxide fluxes in the western Canadian boreal forest: evidence from towers, aircraft and remote sensing. Agric For Meteorol 115:91–107.
Bardgett RD, Wardle DA. 2003. Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–68.
Bardgett RD, Wardle DA. 2010. Aboveground-Belowground Linkages. Biotic Interactions, Ecosystem Processes, and Global Change. Oxford University Press.
Bardgett RD, Marsden JH, Howard DC. 1995. The extent and condition of heather on moorland in the uplands of England and Wales. Biol Conserv 71:155–61.
Bardgett RD, Hobbs PJ, Frostegard A. 1996. Changes in soil fungal: bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol Fertil Soils 22:261–4.
Boutton TW. 1991. Stable carbon isotope ratios of natural materials: II. Atmospheric, terrestrial, marine and freshwater environments. In: Coleman, DC, Ed. Carbon isotope techniques. Academic Press, Inc., pp 173–85.
Bubier JL, Frolking S, Crill PM, Linder E. 1999. Net ecosystem productivity and its uncertainty in a diverse boreal peatland. J Geophys Res Atmospheres 104:27683–92.
Chapin FSIII, Walker BH, Hobbs RJ, Hooper DU, Lawton JH, Sala OE, Tilman D. 1997. Biotic control over the functioning of ecosystems. Science 277:500–4.
Clay GD, Worrall F, Rose R, 2010. Carbon budgets of an upland blanket bog managed by prescribed fire. J. Geophys. Res Biogeosci 115, (G04037), 14. doi:10.1029/2010JG001331.
De Deyn GB, Cornelissen JHC, Bardgett RD. 2008. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol Lett 11:516–31.
De Deyn GB, Quirk H, Oakley S, Ostle N, Bardgett RD. 2011. Rapid transfer of photosynthetic carbon through the plant-soil system in differently managed species-rich grasslands. Biogeosciences 8:1131–9.
DeLuca TH, Zouhar KL. 2000. Effects of selection harvest and prescribed fire on the soil nitrogen status of ponderosa pine forests. For Ecol Manag 138:263–71.
DeLuca TH, Nilsson MC, Zackrisson O. 2002. Nitrogen mineralization and phenol accumulation along a fire chronosequence in northern Sweden. Oecologia 133:206–14.
Dise NB. 2009. Peatland response to global change. Science 326:810–11.
Dorrepaal E, Toet S, van Logtestijn RSP, Swart E, van de Weg MJ, Callaghan TV, Aerts R. 2009. Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 460:616–9.
Eddy A, Welch D, Rawes M. 1969. The vegetation of the Moor House National Nature Reserve in the northern Pennines, England. Vegetatio Acta Geobotanica XVI:28–111.
Farage P, Ball A, McGenity TJ, Whitby C, Pretty J. 2009. Burning management and carbon sequestration of upland heather moorland in the UK. Aust J Soil Res 47:351–61.
Garnett MH, Ineson P, Stevenson AC, Howard DC. 2001. Terrestrial organic carbon storage in a British moorland. Glob Change Biol 7:375–88.
Gorham E. 1991. Northern Peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol Appl 1:182–95.
Grace J, Marks TC. 1978. Physiological aspects of bog production at Moor House. In: Heal OW, Perkins DF, Eds. Production ecology of British moors and montane grasslands. New York: Springer-Verlag. p 38–51.
Greenup AL, Bradford MA, McNamara NP, Ineson P, Lee JA. 2000. The role of Eriophorum vaginatum in CH4 flux from an ombrotrophic peatland. Plant Soil 227:265–72.
Hardie SML, Garnett MH, Fallick AE, Ostle NJ, Rowland AP. 2009. Bomb-C-14 analysis of ecosystem respiration reveals that peatland vegetation facilitates release of old carbon. Geoderma 153:393–401.
Harrison KA, Bardgett RD. 2010. Influence of plant species and soil conditions on plant-soil feedback in mixed grassland communities. J Ecol 98:384–95.
Hart SC, DeLuca TH, Newman GS, MacKenzie MD, Boyle SI. 2005. Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For Ecol Manag 220:166–84.
Heal OW, Smith RAH. 1978. The Moor House programme: introduction and site description. In: Heal OW, Perkins DF, Eds. Production ecology of British moors and montane grasslands. New York: Springer-Verlag. p 1–16.
Heikkinen JEP, Maijanen M, Aurela M, Hargreaves KJ, Martikainen PJ. 2002. Carbon dioxide and methane dynamics in a sub-Arctic peatland in northern Finland. Polar Res 21:49–62.
Hogberg P, Read DJ. 2006. Towards a more plant physiological perspective on soil ecology. Trends Ecol Evol 21:548–54.
Holland EA, Robertson GP, Greenberg J, Groffman PM, Boone RD, Gosz JR. 1999. Soil CO2, N2O, and CH4 exchange. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P, Eds. Standard soil methods for long-term ecological research. LTER.
Hubbard RM, Vose JM, Clinton BD, Elliott KJ, Knoepp JD. 2004. Stand restoration burning in oak-pine forests in the southern Appalachians: effects on aboveground biomass and carbon and nitrogen cycling. For Ecol Manag 190:311–21.
IPCC. 2007. Climate change Reports, WGI, II, III.
Jin VL, Evans RD. 2010. Microbial 13C utilization patterns via stable isotope probing of phospholipid biomarkers in Mojave Desert soils exposed to ambient and elevated atmospheric CO2. Glob Change Biol 16:2334–44.
Kareiva P, Watts S, McDonald R, Boucher T. 2007. Domesticated nature: shaping landscapes and ecosystems for human welfare. Science 316:1866–9.
Kuhry P. 1994. The role of fire in the development of sphagnum-dominated peatlands in western boreal Canada. J Ecol 82:899–910.
Lang SI, Cornelissen JHC, Klahn T, van Logtestijn RSP, Broekman R, Schweikert W, Aerts R. 2009. An experimental comparison of chemical traits and litter decomposition rates in a diverse range of subarctic bryophyte, lichen and vascular plant species. J Ecol 97:886–900.
Leake JR, Ostle NJ, Rangel-Castro JI, Johnson D. 2006. Carbon fluxes from plants through soil organisms determined by field (CO2)-C-13 pulse-labelling in an upland grassland. Appl Soil Ecol 33:152–75.
McNamara NP, Plant T, Oakley S, Ward S, Wood C, Ostle N. 2008. Gully hotspot contribution to landscape methane (CH4) and carbon dioxide (CO2) fluxes in a northern peatland. Sci Total Environ 404:354–60.
Michelsen A, Andersson M, Jensen M, Kjoller A, Gashew M. 2004. Carbon stocks, soil respiration and microbial biomass in fire- prone tropical grassland, woodland and forest ecosystems. Soil Biol Biochem 36:1707–17.
Miles J. 1988. Vegetation and soil change in the uplands. In: Usher MB, Thompson DBA, Eds. Ecological change in the uplands. Blackwell Scientific, pp 57–70.
Nykanen H, Heikkinen JEP, Pirinen L, Tiilikainen K, Martikainen PJ. 2003. Annual CO2 exchange and CH4 fluxes on a subarctic palsa mire during climatically different years. Glob Biogeochem Cycles 17, art. no.-1018.
Ostle N, Ineson P, Benham D, Sleep D. 2000. Carbon assimilation and turnover in grassland vegetation using an in situ (CO2)-C-13 pulse labelling system. Rapid Commun Mass Spectrom 14:1345–50.
Ostle N, Whiteley AS, Bailey MJ, Sleep D, Ineson P, Manefield M. 2003. Active microbial RNA turnover in a grassland soil estimated using a (CO2)-C-13 spike. Soil Biol Biochem 35:877–85.
Ostle NJ, Levy PE, Evans CD, Smith P. 2009. UK land use and soil carbon sequestration. Land Use Policy 26:S274–83.
Rawes M, Hobbs R. 1979. Management of semi-natural blanket bog in the Northern Pennines. J Ecol 67:789–807.
Rodwell JS. 1991. British plant communities. Vol 2. Mires and heaths. Cambridge: Cambridge University Press.
Simmons IG. 2003. The moorlands of England and Wales. An environmental history 8000 BC–AD 2000. Edinburgh University Press, UK.
Trinder CJ, Artz RRE, Johnson D. 2008. Contribution of plant photosynthate to soil respiration and dissolved organic carbon in a naturally recolonising cutover peatland. Soil Biol Biochem 40:1622–8.
Turetsky M, Wieder K, Halsey L, Vitt D. 2002. Current disturbance and the diminishing peatland carbon sink. Geophys Res Lett 29:art. no.-1526.
Updegraff K, Bridgham SD, Pastor J, Weishampel P, Harth C. 2001. Response of CO2 and CH4 emissions from peatlands to warming and water table manipulation. Ecol Appl 11:311–26.
van der Heijden MGA, Bardgett RD, van Straalen NM. 2008. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310.
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. 1997. Human domination of the Earth's ecosystems. Science 277:494–9
Waddington JM, Roulet NT. 2000. Carbon balance of a boreal patterned peatland. Glob Change Biol 6:87–97.
Ward SE, Bardgett RD, McNamara NP, Adamson JK, Ostle NJ. 2007. Long-term consequences of grazing and burning on northern peatland carbon dynamics. Ecosystems 10:1069–83.
Ward SE, Bardgett RD, McNamara NP, Ostle NJ. 2009. Plant functional group identity influences short-term peatland ecosystem carbon flux: evidence from a plant removal experiment. Funct Ecol 23:454–62.
Wardle DA, Bardgett RD, Klironomos JN, Setala H, van der Putten WH, Wall DH. 2004. Ecological linkages between aboveground and belowground biota. Science 304:1629–33.
White DC, Davis WM, Nickels JS, King JD, Bobbie RJ. 1979. Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia 40:51–62.
Wieder RK, Scott KD, Kamminga K, Vile MA, Vitt DH, Bone T, Xu B, Benscoter BW, Bhatti JS. 2009. Postfire carbon balance in boreal bogs of Alberta, Canada. Glob Change Biol 15:63–81.
Woodin SJ, van der Wal R, Sommerkorn M, Gornall JL. 2009. Differential allocation of carbon in mosses and grasses governs ecosystem sequestration: a 13C tracer study in the high Arctic. New Phytologist 184:944–9.
Worrall F, Armstrong A, Adamson JK. 2007. The effects of burning and sheep-grazing on water table depth and soil water quality in a upland peat. J Hydrol 339:1–14.
Yallop AR, Clutterbuck B. 2009. Land management as a factor controlling dissolved organic carbon release from upland peat soils 1: Spatial variation in DOC productivity. Sci Total Environ 407:3803–13.
Acknowledgements
This research was supported by the Natural Environment Research Council (NERC) EHFI grant (NE/E011594/1) awarded to R. D. Bardgett and N. J. Ostle. The authors thank Hannah Tobermann and Emily Bottoms for their help in the field, Helen Grant for her stable isotope analysis, and two anonymous referees for their helpful comments on an earlier versions of this manuscript. The authors also thank Natural England and the Environmental Change Network, CEH, Lancaster for access to and information on the field site.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
SEW, NJO, RDB conceived and designed the study and wrote the paper. SEW, SO, HQ, AS performed the research and contributed methods. SEW, PAH, WAS analysed data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ward, S.E., Ostle, N.J., Oakley, S. et al. Fire Accelerates Assimilation and Transfer of Photosynthetic Carbon from Plants to Soil Microbes in a Northern Peatland. Ecosystems 15, 1245–1257 (2012). https://doi.org/10.1007/s10021-012-9581-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-012-9581-8