Abstract
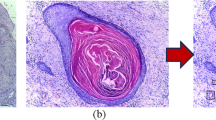
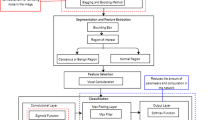
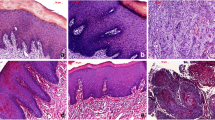
Currently, the diagnoses of oral diseases primarily depend on the visual recognition of experienced clinicians. It has been proven that automatic recognition based on images can support clinical decision-making by extracting and analyzing objective hidden information. In recent years, optical coherence tomography (OCT) has become a powerful optical imaging technique with the advantages of high resolution and non-invasion. In our study, a dataset composed of four kinds of oral salivary gland tumors (SGTs) was obtained from a homemade swept-source OCT, including two benign and two malignant tumors. Seventy-six texture features were extracted from OCT images to create computational models of diseases. It was demonstrated that the artificial neural network (ANN) based on principal component analysis (PCA) can obtain high diagnostic sensitivity and specificity (higher than 99%) for these four kinds of tumors. The classification accuracy of each tumor is larger than 99%. In addition, the performances of two classifiers (ANN and support vector machine) were quantitatively evaluated based on SGTs. It was proven that the texture features in OCT images provided objective information to classify oral tumors.







Similar content being viewed by others
References
Skalova A, Stenman G, Simpson RHW et al (2018) The role of molecular testing in the differential diagnosis of salivary gland carcinomas. Am J Surg Pathol 42(2):e11–e27. https://doi.org/10.1097/PAS.0000000000000980
Palasz P, Adamski L, Gorska-Chrzastek M, Starzynska A, Studniarek M (2017) Contemporary diagnostic imaging of oral squamous cell carcinoma - a review of literature. Pol J Radiol 7(82):193–202. https://doi.org/10.12659/PJR.900892
Li Q, Zhang X, Liu X et al (2012) Long-term treatment outcome of minor salivary gland carcinoma of the hard palate. Oral Oncol 48(5):456–462. https://doi.org/10.1016/j.oraloncology.2011.12.005
Heller KS, Dubner S, Chess Q, Attie JN (1993) Value of fine needle aspiration biopsy of salivary gland mass in clinical decision making. Am J Surg 164(6):667–670. https://doi.org/10.1016/S0002-9610(05)80731-7
Inohara H, Akahani S, Yamamoto Y et al (2008) The role of fine-needle aspiration cytology and magnetic resonance imaging in the management of parotid mass lesions. Acta Otolaryngol 128:1152–1158. https://doi.org/10.1080/00016480701827533
Limongelli L, Capodiferro S, Tempesta A et al (2020) Early tongue carcinomas (clinical stage I and II): echo-guided three-dimensional diode laser mini-invasive surgery with evaluation of histological prognostic parameters. A study of 85 cases with prolonged follow-up. Lasers Med Sci 35(3):751–758. https://doi.org/10.1007/s10103-019-02932-z
Kumar P, Kanaujia SK, Singh A, Pradhan A (2019) In vivo detection of oral precancer using a fluorescence-based, in-house-fabricated device: a Mahalanobis distance-based classification. Lasers Med Sci 34:1243–1251. https://doi.org/10.1007/s10103-019-02720-9
Anschau F, Webster J, Capra MEZ, de Silva ALFD, Stein AT (2019) Efficacy of low-level laser for treatment of cancer oral mucositis: a systematic review and meta-analysis. Lasers Med Sci 34:1053–1062. https://doi.org/10.1007/s10103-019-02722-7
Koch M, Schapher M, Goncalves M, Iro H, Mantsopoulos K (2020) Simultaneous application of ultrasound and sialendoscopy: experience in the management of stenosis and other non-sialolithiasis-related salivary gland disorders. Eur Rev Med Pharmaco 24(5):2196–2204. https://doi.org/10.26355/eurrev_202003_20485
Razek A (2019) Multi-parametric MR imaging using pseudo-continuous arterial-spin labeling and diffusion-weighted MR imaging in differentiating subtypes of parotid tumors. Magn Reson Imaging 63:55–59. https://doi.org/10.1016/j.mri.2019.08.005
Wu W, Wang C, Li D, Luo J, Ye J, Xu S (2019) Multiphase contrast-enhanced computed tomography imaging features of salivary duct carcinoma: differentiation from other salivary gland malignancies. Oral Surg Oral Med Oral Pathol Oral Radiol 128(5):543–551. https://doi.org/10.1016/j.oooo.2019.05.011
Fakhry N, Antonini F, Michel J et al (2012) Fine-needle aspiration cytology in the management of parotid masses: evaluation of 249 patients. Eur Ann Otorhinolaryngol Head Neck Dis 129(3):131–135. https://doi.org/10.1016/j.anorl.2011.10.008
Alnawaiseh M, Schubert F, Heiduschka P, Eter N (2019) Optical coherence tomography angiography in patients with retinitis pigmentosa. Retina 39(1):210–217. https://doi.org/10.1097/IAE.000000000000190
Turani Z, Fatemizadeh E, Blumetti T et al (2019) Optical radiomic signatures derived from optical coherence tomography images to improve identification of melanoma. Cancer Res 79(8):2021–2030. https://doi.org/10.1158/0008-5472.CAN-18-2791
Jerjes W, Hamdoon Z, Hopper C (2019) Structural validation of facial skin using optical coherence tomography: a descriptive study. Skin Res Technol 00:1–10. https://doi.org/10.1111/srt.12791
Yonetsu T, Bouma BE, Kato K, Fujimoto JG, Jang IK (2013) Optical coherence tomography - 15 years in cardiology. Circ J 77(8):1933–1940. https://doi.org/10.1253/circj.cj-13-0643.1
Gan Y, Tsay D, Amir SB, Marboe CC, Hendon CP (2016) Automated classification of optical coherence tomography images of human atrial tissue. J Biomed Opt 21(10):101407
Kut C, Chaichana KL, Xi J et al (2015) Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci Transl Med 7(292):292ra100. https://doi.org/10.1126/scitranslmed.3010611
Erickson-Bhatt SJ, Mesa KJ, Marjanovic M, Chaney EJ, Ahmad A, Huang P-C, Liu ZG, Cunningham K, Boppart SA (2018) Intraoperative optical coherence tomography of the human thyroid: feasibility for surgical assessment. Transl Res 195:13–24. https://doi.org/10.1016/j.trsl.2017.12.001
Yang Z, Shang J, Liu C, Zhang J, Liang Y (2020) Identification of oral cancer in OCT images based on an optical attenuation model. Lasers Med Sci 35:1999–2007. https://doi.org/10.1007/s10103-020-03025-y
Hamdoon Z, Jerjes W, Upile T, McKenzie G, Jay A, Hopper C (2013) Optical coherence tomography in the assessment of suspicious oral lesions: an immediate ex vivo study. Photodiagn Photodyn 10(1):17–27. https://doi.org/10.1016/j.pdpdt.2012.07.005
Walther J, Golde J, Kirsten L et al (2017) In vivo imaging of human oral hard and soft tissues by polarization-sensitive optical coherence tomography. J Biomed Opt 22(12):121717. https://doi.org/10.1117/1.JBO.22.12.121717
Wang J, Zheng W, Lin K, Huang Z (2018) Characterizing biochemical and morphological variations of clinically relevant anatomical locations of oral tissue in vivo with hybrid Raman spectroscopy and optical coherence tomography technique. J Biophotonics 11(3):e201700113. https://doi.org/10.1002/jbio.201700113
Yang Z, Shang J, Liu C, Zhang J, Hou F, Liang Y (2020) Intraoperative imaging of oral-maxillofacial lesions using optical coherence tomography. J Innov Opt Heal Sci 13(2):2050010. https://doi.org/10.1142/s1793545820500108
Li K, Yang Z, Liang W, Shang J, Liang Y, Wan S (2020) Low-cost, ultracompact handheld optical coherence tomography probe for in vivo oral maxillofacial tissue imaging. J Biomed Opt 25(4):046003. https://doi.org/10.1117/1.JBO.25.4.046003
Koprowski R, Teper S, Wróbel Z, Wylegala E (2013) Automatic analysis of selected choroidal diseases in OCT images of the eye fundus. Biomed Eng OnLine 12(1):117. https://doi.org/10.1186/1475-925X-12-117
Fu D, Tong H, Zheng S, Luo L, Gao F, Minar J (2016) Retinal status analysis method based on feature extraction and quantitative grading in OCT images. Biomed Eng Online 15:87. https://doi.org/10.1186/s12938-016-0206-x
Alsaih K, Lemaitre G, Rastgoo M, Massich J, Sidibe D, Meriaudeau F (2017) Machine learning techniques for diabetic macular edema (DME) classification on SD-OCT images. Biomed Eng Online 16:68. https://doi.org/10.1186/s12938-017-0352-9
Wan S, Lee HC, Huang X et al (2017) Integrated local binary pattern texture features for classification of breast tissue imaged by optical coherence microscopy. Med Image Anal 38:104–116. https://doi.org/10.1016/j.media.2017.03.002
Adabi S, Hosseinzadeh M, Noei S et al (2017) Universal in vivo textural model for human skin based on optical coherence tomograms. Sci Rep 7(1):17912. https://doi.org/10.1038/s41598-017-17398-8
Lenz M, Krug R, Dillmann C et al (2018) Automated differentiation between meningioma and healthy brain tissue based on optical coherence tomography ex vivo images using texture features. J Biomed Opt 23(7):071205. https://doi.org/10.1117/1.JBO.23.7.071205
Hou F, Yu Y, Liang Y (2017) Automatic identification of parathyroid in optical coherence tomography images. Lasers Surg Med 49(3):305–311. https://doi.org/10.1002/lsm.22622
Bian D, Tao K, Yuan Z, Kuang H, Liu Z, Liang Y (2020) Identification of atherosclerotic plaques in intravascular optical coherence tomography images based on textures and artificial neural network. Laser Phys 30(3):035602. https://doi.org/10.1088/1555-6611/ab7239
Ou X, Pan W, Xiao P (2014) In vivo skin capacitive imaging analysis by using grey level co-occurrence matrix (GLCM). Int J Pharm 460:28–32. https://doi.org/10.1016/j.ijpharm.2013.10.024
Lingley-Papadopoulos CA, Loew MH, Manyak MJ, Zara JM (2008) Computer recognition of cancer in the urinary bladder using optical coherence tomography and texture analysis. J Biomed Opt 13(2):024003. https://doi.org/10.1117/1.2904987
Govindaraj P, Sudhakar MS (2017) Shape characterization using laws of texture energy measures facilitating retrieval. Imaging Sci J 66:98–105. https://doi.org/10.1080/13682199.2017.1380356
Qi X, Sivak MV Jr, Isenberg G, Willis JE, Rollins AM (2006) Computer-aided diagnosis of dysplasia in Barrett’s esophagus using multiple endoscopic OCT images. J Biomed Opt 11(4):044010. https://doi.org/10.1117/1.2337314
Ahonen T, Hadid A, Pietikäinen M (2004) Face recognition with local binary patterns. Computer vision - ECCV pp 469–481. https://doi.org/10.1007/978-3-540-24670-1_36
Keerthi Vasan K, Surendiran B (2016) Dimensionality reduction using principal component analysis for network intrusion detection. Perspectives in Science 8:510–512. https://doi.org/10.1016/j.pisc.2016.05.010
Acknowledgements
This study has been funded by the National Natural Science Foundation of China (61875092), Science and Technology Support Program of Tianjin (17YFZCSY00740), and the Beijing-Tianjin-Hebei Basic Research Cooperation Special Program (19JCZDJC65300).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Ethics Committee of Tianjin Stomatological Hospital.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Z., Shang, J., Liu, C. et al. Classification of oral salivary gland tumors based on texture features in optical coherence tomography images. Lasers Med Sci 37, 1139–1146 (2022). https://doi.org/10.1007/s10103-021-03365-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03365-3