Abstract
Background
The sentinel node (SN) concept is safely applied and validated in early gastric cancer. Gastric lymph nodes are divided into five basins with the main gastric arteries, and the anterosuperior lymph nodes with the common hepatic artery (No. 8a) are classified in the right gastric artery (r-GA) basin. Although No. 8a are considered to have lymphatic flow from the r-GA basin, there might be additional multiple lymphatic flows into No. 8a. The aim of this study is to analyze the lymphatic flows to No. 8a and to investigate the clinical significance of No. 8a as a sentinel node (SN No. 8a).
Methods
Four hundred and twenty-nine patients with cT1N0 or cT2N0 gastric cancer underwent SN mapping. We used technetium-99 tin colloid solution and blue dye as a tracer.
Results
We detected SN No. 8a in 35 (8.2 %) patients. In these patients, we detected SN No. 8a with SNs that belonged to the left gastric artery (l-GA) basin (66 %), right gastroepiploic artery (r-GEA) basin (54 %), and right gastric artery (r-GA) basin (46 %). In addition, celiac artery lymph nodes were detected as SNs significantly more frequently. Function-preserving surgery was performed significantly less often in patients with SN No. 8a (p =0.018).
Conclusions
We found that SN No. 8a seemed to have lymphatic flow not only from the r-GA basin, but also from the l-GA basin or r-GEA basin. When SN No. 8a are detected, we should be careful to perform function-preserving surgery, even in SN-negative cases.
Similar content being viewed by others
Introduction
A sentinel node (SN) is defined as the first draining lymph node from a primary tumor. According to the SN concept, when SN is negative for metastasis, metastasis to the other lymph nodes will also be negative. The SN concept has already been applied clinically in the fields of melanoma [1, 2] and breast cancer [3]. The application of this concept benefits patients by preventing unnecessary prophylactic regional lymph node dissection.
Currently, in Asian countries, early gastric cancer is diagnosed in many asymptomatic patients, because of improvements in endoscopic technology and the promotion of preventive medicine. In Japan and Korea, early gastric cancer accounts for 50 % of newly diagnosed gastric cancer [4]. The standard treatments require gastrectomy with D1 or D2 lymphadenectomy, although the incidence of regional lymph node metastasis is limited in patients with early gastric cancer [5]. Therefore, it has long been hoped that the SN concept would be applied to early gastric cancer in order to avoid unnecessary prophylactic lymph node dissection.
Some single-institution studies have reported the feasibility of SN mapping for gastric cancer, and the SN concept has been thought to be acceptable in early gastric cancer [6–9]. Recently, a study group of the Japan Society of Sentinel Node Navigation Surgery conducted a multicenter trial and reported the validity of SN mapping in patients with early gastric cancer [10].
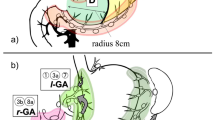
Several studies have proposed the concept of SN basin dissection [11, 12]. The gastric lymphatic compartments are divided into five SN basins with the main gastric arteries as follows: left gastric artery (l-GA) basin, right gastric artery (r-GA) basin, right gastroepiploic artery (r-GEA) basin, left gastroepiploic artery (l-GEA) basin, and posterior gastric artery (p-GA) basin.
The stomach lymph nodes are defined and given station numbers anatomically in the Japanese classification of gastric carcinoma [13]. Lymph nodes 8a (LN No. 8a) are defined as the anterosuperior lymph nodes along the common hepatic artery [13]. According to Kinami et al. [12], LN No. 8a are considered to belong to the r-GA basin. LN No. 8a are also defined as a secondary compartment of lymph nodes in the Japanese classification [13]; therefore, LN No. 8a may have lymphatic flow from other lymphatic basins. However, no studies have reported in which SN basins LN No. 8a are exactly included or whether it is really correct to classify LN No. 8a in the r-GA basin. We hypothesized that LN No. 8a receive lymphatic influx not only from the r-GA basin but also from other SN basins. In this study, we retrospectively analyzed lymphatic flows of patients who underwent SN mapping for gastric cancer in our institution and patients in whom LN No. 8a were detected as SNs.
Patients and methods
Patients
From January 1999 to December 2011, 429 patients who were diagnosed with cT1N0 or cT2N0 gastric cancer with a single primary lesion, underwent gastric surgery with SN mapping in Keio University Hospital. Clinical staging was made by preoperative endoscopy and computed tomography, according to the Japanese Classification of Gastric Cancer, which was proposed by the Japanese Gastric Cancer Association [13], and the TNM classification [14]. Although we reported recently that the SN concept may be applicable in patients after endoscopic treatment, the validity and accuracy of SN biopsy after endoscopic treatment have not been fully investigated [15]. Therefore, we excluded patients from this study who previously had endoscopic treatment and who required additional gastrectomy.
Methods
We used the double-tracer method with radioactive colloid and blue dye to detect SNs, as described previously in detail [9]. The day before surgery, we injected a 2.0-ml (150 MBq) solution of technetium-99 m tin colloid in four quadrants of the submucosal layer surrounding the primary cancer lesion using an endoscopic puncture needle. Technetium-99 m tin colloid migrates into SNs within 2 h after injection, and remains there for more than 20 h through phagocytosis by macrophages [9]. Indocyanine green (ICG) or isosulfan blue was used as a blue dye, which we injected by intraoperative endoscopy using the same method used for injection of the radioactive colloid. Within 15 min, we were able to visualize blue-stained vessels and lymph nodes. Simultaneously, we used a hand-held gamma probe (GPS navigator; RMD Instruments, Watertown, MA, USA) to detect radioactive SNs. We diagnosed lymph nodes with radioactivity >10 × background activity or those that were blue stained as “hot nodes.”
We subjected all the detected SNs to intraoperative histological examination. After performing the SN examination, we decided on the surgical procedure according to the results of intraoperative histological examination of SN metastasis. If metastasis of SNs was negative by intraoperative pathological diagnosis, we considered function-preserving surgery (pylorus-preserving gastrectomy or proximal gastrectomy). If it was difficult to perform function-preserving surgery, we performed D1 or D2 gastrectomy (distal gastrectomy or total gastrectomy) according to the Japanese guidelines of gastric cancer treatment [5]. If metastasis to SNs was positive, we performed D2 gastrectomy (distal gastrectomy or total gastrectomy).
This study was approved by the Institutional Review Board of Keio University School of Medicine, and all patients provided written informed consent for the whole procedure of SN mapping before the operation.
Statistical analysis
We performed statistical analyses using SPSS statistics, version 22 (IBM, Armonk, NY, USA). We analyzed the clinical and pathological variables using the χ 2 test and Fisher’s exact tests. We considered differences to be statistically significant at p < 0.05.
Results
The patient characteristics are described in Table 1. Of all 429 gastric cancer patients who underwent SN mapping, we detected lymph nodes No. 8a (LN No. 8a) as SNs in 35 patients (8.2 %) (the SN No. 8a group). We did not detect SN No. 8a in the remaining 394 patients, and we defined those patients as the control group. There was no significant difference in patient characteristics between these two groups (Table 1).
In the SN No. 8a group, primary tumor lesions were mainly located in the middle third (60 %) or the lower third (37 %) of the gastric body. In the middle third location, primary lesions were circumferentially seen (anterior wall 33 %, posterior wall 19 %, lesser curvature wall 29 %, greater curvature wall 19 %, of all SN No. 8a group patients), whereas in the lower third location, primary lesions were mainly seen in the lesser curvature side (anterior wall 15 %, posterior wall 23 %, lesser curvature wall 46 %, greater curvature wall 15 %, of all SN No. 8a group patients) of the stomach.
Data regarding the surgical procedures and pathological findings are shown in Table 2. In the SN No. 8a group, we performed standard gastrectomy (distal gastrectomy and total gastrectomy) in 31 patients (88 %) and function-preserving gastrectomy (proximal gastrectomy and pylorus-preserving gastrectomy) in 4 patients (12 %). Function-preserving gastrectomy was performed less often in the SN No. 8a group (p = 0.018). Of all these surgical procedures, we performed laparoscopy-assisted gastrectomy in 292 patients and open gastrectomy in 137 patients, which was not significantly different between the SN No. 8a group and the control group (p = 0.947).
There were no significant differences between the two groups in the pathological findings (Table 2). In the SN No. 8a group, we detected SN metastases in 4 cases (11 % of all the SN No. 8a group patients), and SN No. 8a metastases in 2 cases (6 % of all the SN No. 8a group patients). However, there was no significant difference in the proportion of patients with SN metastases between the SN No. 8a group and control group (p = 0.930).
The distribution of SNs and SN basins detected with SN No. 8a is shown in Fig. 1. With regard to the distribution of SNs in the SN No. 8a group, SNs detected with SN No. 8a mainly belonged to the l-GA basin (66 %), r-GEA basin (54 %), and r-GA basin (46 %). Although SN No. 8a are classified in the r-GA basin, SNs detected with SN No. 8a did not belong to the r-GA basin in 19 patients (54 %). The distribution of the main three SN basins (l-GA, r-GEA, r-GA) in detail was as follows: l-GA + r-GA (29 %), r-GEA alone (26 %), l-GA + r-GA + r-GEA (14 %), l-GA alone (14 %), l-GA + r-GEA (11 %), and r-GA + r-GEA (3 %); SN No. 8a was the only detected SN in 1 case (3 %). With regard to single lymph node stations detected with SN No. 8a, SN No. 9 was detected significantly more often in the SN No. 8a group (p = 0.008). In 5 cases with SN No. 9 (11 % of all the SN No. 8a group patients), the tumor locations were as follows: 2 cases on the lesser curvature side in the lower third of the stomach, 1 case on the greater curvature side in the upper third of the stomach, 1 case on the lesser curvature side in the middle third of the stomach, and 1 case on the posterior wall in the lower third of the stomach.
Distribution of SNs and SN basins detected with SN No. 8a. SNs detected with SN No. 8a were mainly Nos. 3a (54 %), 3b (51 %), 4d (43 %), 7 (29 %), and 6 (23 %). No. 9 were detected significantly more often in the SN No. 8a group (p = 0.008). The SN basins detected with SN No. 8a were l-GA basin (66 %), r-GEA basin (54 %), and r-GA basin (46 %)
Discussion
In Kinami’s classification [12], SN No. 8a are classified in the r-GA basin, and the lymphatics are thought to come from the same flow as lymph nodes No. 3b (i.e., lesser curvature lymph nodes along the second branch and distal part of the right gastric artery) and lymph nodes No. 5 (i.e., suprapyloric lymph nodes along the first branch and proximal part of the right gastric artery). However, according to our results, SN No. 8a were detected with other SNs, except for one case, and in more than half of the cases in the SN No. 8a group, SN No. 8a were detected with SNs classified to the l-GA or r-GEA basin. Our results suggest the possibility of multiple lymphatic flows from the l-GA or r-GEA basin to SN No. 8a. With regard to the single lymph node station, lymph nodes No. 9 were detected as SNs significantly more in the SN No. 8a group. This result suggests that there was lymphatic flow between lymph nodes Nos. 8a and 9.
In addition, in the SN No. 8a group, function-preserving surgery was performed significantly less frequently. Because we detected SNs in the l-GA basin or r-GEA basin in addition to the r-GA basin, it was technically difficult to perform function-preserving surgery. Regardless of the fact that SNs belonged to more than one SN basin in patients with SN No. 8a, SN metastasis occurrence did not differ between patients in the SN No. 8a group and the control group.
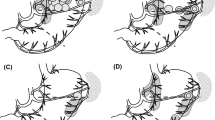
Considering our results, it seems difficult to classify SN No. 8a in a single SN basin, because SN No. 8a may consist of lymphatic flows from several basins. LN No. 8a are defined as lymph nodes covering a wide region from a branch of the splenic artery to a branch of the gastroduodenal artery, and many unknown lymphatics may converge in this area. Deki and Sato [16] previously reported, from their anatomical study, that there were lymphatic pathways from the head of the pancreas toward the common hepatic artery. This finding suggests the anatomical existence of the lymphatic flow from the proximal part of the right gastroepiploic artery toward LN No. 8a. Recently, ICG fluorescence imaging has been attracting attention, and it is expected to be a novel tool to detect SNs [17, 18]. Tajima et al. [19, 20] reported on intraoperative ICG fluorescence imaging using a charge-coupled device camera with a light-emitting diode (Hamamatsu Photonics, Hamamatsu, Japan). Using this technique, not only SNs but also lymph vessels can be visualized in a timely manner. We think this visual information will be important to analyze lymphatics. Currently, we have been using ICG fluorescence imaging to visualize lymphatic pathways in addition to detecting SN with radioisotope and blue dye [9, 21]. We have observed a case in which we visualized lymphatic pathways from the r-GEA or l-GA basin toward the direction of LN No. 8a (Fig. 2). This result may support our hypothesis that there is a lymphatic pathway from the r-GEA basin and l-GA basin toward LN No. 8a. However, we need to accumulate more cases for further elucidation of unknown lymphatic streams in gastric cancer patients and to show the feasibility of ICG fluorescence imaging.
These pictures represent an early gastric cancer patient with a tumor lesion on the lesser curvature side of the lower third of the stomach (a schematic of the case). In this patient, SN Nos. 5, 6, 7, and 8a were detected. Red arrowheads show the lymphatic flow that was visualized by near-infrared imaging. We detected lymphatic flow from the proximal part of the right gastric artery toward SN No. 8a (b overview of the image, c indocyanine green fluorescence image), the lymphatic flow from the proximal part of the right gastroepiploic artery toward SN No. 8a (d overview of the image, e indocyanine green fluorescence image), and the lymphatic flow along left gastric artery toward SN No. 8a (f overview of the image, g near-infrared image)
Several retrospective studies have reported that function-preserving surgery improves the postoperative quality of life, and that it can be safely performed [22–26]. On the other hand, a recent meta-analysis by Mocellin et al. [27] showed that D2 lymphadenectomy can improve disease-specific survival in patients with resectable carcinoma of the stomach. We cannot underestimate the possibility of lymph nodes metastasis for cT1 gastric cancer patients. The results from the multicenter prospective trial demonstrated that the SN concept is a feasible and safe method for patients at risk for lymph node metastasis [10]. If we could precisely classify these patients by SN mapping, D2 lymphadenectomy may be considered excessive for some patients. The phase III multicenter trial, which compares standard gastrectomy with minimized gastrectomy combined with SN mapping, is now in progress in Korea [28]. We also cannot ignore the possibility of micro-metastasis, as mentioned in previous studies [29, 30]. It is suggested that even in the SN metastasis-negative cases, not the pickup of SNs but SN basin dissection is necessary to overcome the risk of false-negative cases [10]. We adopted SN basin dissection for SN metastasis-negative cases in our study. The indication for SN mapping is suggested to be cT1N0 from the results of the multicenter trial [10]; however, our study had also included cT2N0 patients. There remains the possibility that the risk of micro-metastasis may be higher in our study, although no recurrence has been detected among all SN-negative cases so far. We suggest that function-preserving surgery can be safely performed for all SN-negative cases.
According to our study, it may be difficult to perform function-preserving surgery when we detect SN No. 8a, but we may consider function-preserving surgery if it is technically possible. In our study, SNs detected with SN No. 8a belonged to a single basin in 14 cases, either in l-GA (5 cases) or r-GEA (9 cases) basin. In these cases, SN No. 8a may have lymphatics from either l-GA or r-GEA basin, but lymphatics from r-GA basin still may be responsible for the lymphatics of SN No. 8a. As long as LN No. 8a are composed of several lymphatics, we should always confirm which SN basin is responsible for SN No. 8a; otherwise, we should decide not to perform function-preserving surgery, even in SN-negative cases.
In addition, we should not forget the fact that in some cases SNs were detected in lymph nodes Nos. 9, 11p, and 14v, and SN No. 9 were found significantly more often in the SN No. 8a group. When SN No. 8a are detected, it is important to ensure that no more SNs remain in the other sentinel basins, including regions beyond the sentinel basins. To ensure this, we recommend that lymph nodes No. 9 should be dissected when SN No. 8a are detected.
In conclusion, when SN No. 8a are detected in early gastric cancer, it is important to carefully investigate for other SNs regardless of the tumor lesion. We showed that SN No. 8a had lymphatic flow not only from r-GA basin but also from l-GA or r-GEA basin. Moreover, if SN No. 8a are detected, we cannot be too careful when performing function-preserving surgery, unless we have sufficient information on the lymphatics of SN No. 8a.
References
Wong JH, Cagle LA, Morton DL. Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann Surg. 1991;214:637–41.
Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–9.
Guiliano AE, Kirigan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–8.
Japanese Gastric Association Registration Committee, Maruyama K, Kaminishi M, Hayashi K, Isobe Y, Honda I, Katai H et al. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006;2006(9):51–66.
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113–23.
Morita D, Tsuda H, Ichikura T, Kimura M, Aida S, Kosuda S, et al. Analysis of sentinel node involvement in gastric cancer. Clin Gastroenterol Hepatol. 2007;5:1046–52.
Ohdaira H, Nimura H, Mitsumori N, Takahashi N, Kashiwagi H, Yanaga K. Validity of modified gastrectomy combined with sentinel node navigation surgery for early gastric cancer. Gastric Cancer. 2008;10:117–22.
Kitagawa Y, Kitano S, Kubota T, Kumai K, Otani Y, Saikawa Y, et al. Minimally invasive surgery for gastric cancer-toward a confluence of two major streams: a review. Gastric Cancer. 2005;8:103–10.
Takeuchi H, Kitagawa Y. New sentinel node mapping technologies for early gastric cancer. Ann Surg Oncol. 2013;20:522–32.
Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704–10.
Miwa K, Kinami S, Taniguchi K, Fushida S, Fujimura T, Nonomura A. Mapping sentinel nodes in patients with early-stage gastric carcinoma. Br J Surg. 2003;90:178–82.
Kinami S, Fujimura T, Ojima E, Fushida S, Ojima T, Funaki H, et al. PTD classification: proposal for a new classification of gastric cancer location based on physiological lymphatic flow. Int J Clin Oncol. 2008;13:320–9.
Japanese classification of gastric carcinoma, 3rd English edition. Gastric Cancer 2011;14:101–12.
UCC. TNM classification of malignant tumors. Weinheim: Wiley; 2009.
Mayanagi S, Takeuchi H, Kamiya S, Niihara M, Nakamura R, Takahashi T, et al. Suitability of sentinel node mapping as an index of metastasis in early gastric cancer following endoscopic resection. Ann Surg Oncol. 2014;21:2987–93.
Deki H, Sato T. An anatomic study of the peripancreatic lymphatics. Surg Radiol Anat. 1988;10:121–5.
Miyashiro I, Miyoshi N, Hiratsuka M, Kishi K, Yamada T, Ohue M, et al. Detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging: comparison with infrared imaging. Ann Surg Oncol. 2008;15:1640–3.
Schaafsma BE, Mieog JS, Hutteman M, van der Vorst JR, Kuppen PJ, Lowik CW, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011;104:323–32.
Tajima Y, Yamazaki K, Masuda Y, Kato M, Yasuda D, Aoki T, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging in gastric cancer. Ann Surg. 2009;249:58–62.
Tajima Y, Murakami M, Yamazaki K, Masuda Y, Kato M, Sato A et al. Sentinel node mapping guided by indocyanine green fluorescence imaging during laparoscopic surgery in gastric cancer. Ann Surg Oncol. 2010;17:1787–93.
Goto O, Takeuchi H, Kawakubo H, Sasaki M, Matsuda T, Matsuda S, et al. First case of non-exposed endoscopic wall-inversion surgery with sentinel node basin dissection for early gastric cancer. Gastric Cancer. 2015;18:434–9.
Morita S, Katai H, Saka M, Fukagawa T, Sano T, Sasako M. Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg. 2008;95:1131–5.
Nunobe S, Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Symptom evaluation of long-term postoperative outcomes after pylorus-preserving gastrectomy for early gastric cancer. Gastric Cancer. 2007;10:167–72.
Saikawa Y, Otani Y, Kitagawa Y, Yoshida M, Wada N, Kubota T, et al. Interim results of sentinel node biopsy during laparoscopic gastrectomy: possible role in function-preserving surgery for early cancer. World J Surg. 2006;30:1962–8.
Takeuchi H, Oyama T, Kamiya S, Nakamura R, Takahashi T, Wada N, et al. Laparoscopy-assisted proximal gastrectomy with sentinel node mapping for early gastric cancer. World J Surg. 2011;35:2463–71.
Suh YS, Han DS, Kong SH, Kwon S, Cl Shin, Kim WH, et al. Laparoscopy-assisted pylorus-preserving gastrectomy is better than laparoscopy-assisted distal gastrectomy for middle-third early gastric cancer. Ann Surg. 2014;259:485–93.
Mocellin S, McCulloch P, Kazi H, Gama-Rodrigues JJ, Yuan Y, Nitti D. Extent of lymph node dissection for adenocarcinoma of the stomach. Cochrane Database Syst Rev. 2015. doi:10.1002/14651858.CD001964.
Ryu K. Future perspective of laparoscopic surgery for gastric cancer: sentinel node navigation function-preserving surgery for early gastric cancer. Transl Gastrointest Cancer. 2013;2:160–3.
Huang JY, Xu YY, Li M, Sun Z, Zhu Z, Song YX, et al. The prognostic impact of occult lymph node metastasis in node-negative gastric cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20:3927–34.
Li Y, Du P, Zhou Y, Cheng Q, Chen D, Wang D, et al. Lymph node micrometastases is a poor prognostic factor for patients in pN0 gastric cancer: a meta-analysis of observational studies. J Surg Res. 2014;191:413–22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
All procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Conflict of interest
Yuko Kitagawa has received grants from Daiichi Sankyo Co., Ltd., Covidien, Taiho Pharmaceutical Co., Ltd., Olympus Corporation, and Nihon Medi-Physics Co., Ltd. Masahiro Jinzaki has received grants from Daiichi Sankyo Co., Ltd.
Rights and permissions
About this article
Cite this article
Shimada, A., Takeuchi, H., Kamiya, S. et al. Clinical significance of the anterosuperior lymph nodes along the common hepatic artery identified by sentinel node mapping in patients with gastric cancer. Gastric Cancer 19, 1088–1094 (2016). https://doi.org/10.1007/s10120-015-0563-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-015-0563-3