Abstract
Objective
Helicobacter pylori (HP) is known to play an important role in the development of gastric cancer (GC). The aim of this study was to analyze the effect of HP eradication on the survival rate and cancer recurrence in patients who underwent subtotal gastrectomy for GC.
Design
Totally 1,031 patients diagnosed with gastric adenocarcinoma who received surgical treatment at the Seoul National University Bundang Hospital from 2003 to 2017 and positive for HP infection were analyzed. The overall and GC-related survival according to HP eradication were compared; risk factors for GC-specific death and cancer recurrence were analyzed, and propensity score matching (PSM) was performed.
Results
Statistically significant benefits of overall and GC-specific survival were observed in the eradicated group compared to the non-eradicated group (P < 0.001), and these benefits were maintained after PSM (P < 0.001) in both of early and advance stage. In Cox proportional hazards multivariate analyses, cancer stage (stage II, adjusted hazard ratio [aHR] = 9.33, P < 0.001; stage III or IV, aHR = 26.17, P < 0.001), and HP positivity (aHR = 3.41, P = 0.001) were independent risk factors for GC-specific death; cancer stage (cancer stage II, aHR = 7.08, P < 0.001; cancer stage III or IV, aHR = 19.64, P < 0.001) and HP positivity (aHR = 2.70; P = 0.005) were independent risk factors for cancer recurrence.
Conclusion
Our results suggest that HP needed to be conducted more intensively in patients who are surgically treated for GC, regardless of cancer stage.
Similar content being viewed by others
Introduction
Helicobacter pylori (HP) is known to play an important role in the development of gastric cancer (GC) [1, 2], however, the effects of HP on the survival of patients with GC have not yet been clarified. Some studies have reported poorer outcomes in patients who were HP negative after gastrectomy [3,4,5]. In these studies, HP-negative status was an independent predictive factor for a poor prognosis in overall survival [3, 5], GC-specific survival [4], and relapse-free survival [3]. However, it is widely accepted that the eradication of HP reduces the recurrence of tumors and improves survival in patients who underwent endoscopic resection for early gastric cancer (EGC) [6, 7]; thus, HP eradication treatment after endoscopic treatment of EGC is recommended in most guidelines. In addition, in the presence of premalignant lesions of GC such as atrophic gastritis and intestinal metaplasia, HP infection has been reported to increase the risk of GC by 4–6 times compared to patients who were HP negative [8, 9], and HP eradication leads to the improvement of premalignant conditions after gastrectomy [10, 11]. However, a significant survival benefit from HP eradication in patients who underwent gastrectomy for GC has not been shown to date. This is due to the difficulty of performing an analysis in a big cohort of patients with GC which is very heterogenous, and GC survival is mainly determined by the TNM classification among patients with advanced gastric cancer (AGC). Thus HP eradication is strongly recommended only in patients with EGC, so far. However, if both of medical cohort and surgical cohort regarding EGC and AGC patients are prospectively collected for their treatments regarding surgery, chemotherapy and HP eradication from early 2003 then it migt be valuable to analyze the effect of HP eradication treatment on survival of GC regardless of cancer stage.
A clinical data warehouse (CDW) is a system developed for the utilization of electronic medical records (EMR) in further data analysis and index monitoring. It has been proven to be powerful and effective, especially with regard to long-term follow-up. The Seoul National University Bundang Hospital (SNUBH) which has been opened in 2003 has been using a comprehensive in-house EMR from beginning [12]; thus, its CDW has been widely used for medical research along with the Korea National Health Insurance Service (NHIS) claims database [13,14,15,16,17]. In addition, SNUBH is the leading hospital for GC treatment and HP eradication with multidisciplinary approach from 2003. From this background, we hypothesized that a significant benefit of HP eradication in the patients with GC who underwent gastrectomy could be proven in terms of overall and GC-specific survival using the CDW of SNUBH along with surgical and medical cohort. Finally, the aim of this study was to investigate the effect of HP eradication on the survival rate and cancer recurrence in patients who underwent surgical resection for GC.
Methods
Study population
Initially, 3,025 patients with > 18 years of age were chosen from prospective surgical cohort who were diagnosed with gastric adenocarcinoma and underwent surgical treatment at SNUBH from 2003 to 2017, and who were tested for HP (Fig. 1). Among them, 679 patients have been enrolled as a prospective medical cohort by NK [18, 19]. The following patients were excluded; patients who underwent total gastrectomy, patients who received only palliative surgery, patients who were lost to follow-up, and patients who had an uncertain HP status [20]. Patients who were initially negative for HP or patients who were spontaneously converted to HP negative during follow-up without eradication and who had a history of HP eradication therapy prior to surgery were excluded for clear data analysis (Fig. 1). Patients who did not follow the HP eradication protocol (Supplementary Fig. 1, see online), that is, received HP eradication 2 years or more after surgery were also excluded. Finally, 1031 patients with HP positivity and follow-up data were selected for the analysis (Fig. 1). Medical records of these patients including death, recurrence of GC, histologic type of cancer, surgical methods used, and social history such as the family history of GC, sex, age, and alcohol and smoking history were collected and reviewed using the CDW as well as surgical and medical cohort. The dates and causes of death of the enrolled patients were cross-reviewed with data from the National Statistical Office for verification. The study was reviewed and approved by the Institutional Review Board (IRB) of SNUBH (IRB number B-1902–523-107), and registered at clinicaltrials.com (NCT 03978481).
HP status evaluation
The HP infection status was determined by histologic examination with the Giemsa staining method, the rapid urease test (CLOtest, Delta West Ltd., Bentley, WA Australia) and/or culture from the antrum and body, respectively, when GC was diagnosed by biopsy [21]. The diagnosis of GC was confirmed again histologically with removed surgical specimen. Postoperatively, the HP test using Giemsa staining and the rapid urease test were performed from the biopsies taken from the lesser curvature (one piece) and the greater curvature (one piece) of the mid-body of remained stomach, respectively, and both of lesser curvature (one piece) and the greater curvature (one piece), together to increase the chance of HP detection. Patients with one or more positive results were confirmed as having an HP-positive infection. Patients with all three negative results were regarded as HP negative and excluded from the analysis. In addition, some cases who showed dynamic changes of HP status, especially in the presence of intestinal metaplasia [20, 22] were also excluded from this analysis.
HP eradication therapy and follow-up
Patients who were diagnosed with GC and received gastrectomy were followed-up annually after surgery by esophagogastroduodenoscopy (EGD), tested for HP at their first follow-up EGD after surgery, and recommended HP eradication routinely in case of HP positive histology result. Patients were recommended to receive anti-HP therapy and patients decided freely to take them or not, as there was no strict guideline for HP-eradication after surgery for GC the recommendation was not so strong until 2017. If the patients refused to receive the HP eradication therapy initially the patients had several opportunities to take anti-HP therapy when the HP tests were positive during regular check-up gastroscopy. When adjuvant chemotherapy was started which took about 6 months then the patients took anti-HP therapy after the completion of schedule. Finally, HP-positive patients who did not treat for HP or received HP eradication within 2 years after surgery were included, and patients who had a history of HP eradication therapy prior to surgery or received HP eradication 2 years or more after surgery were excluded to avoid immortal time bias for survival analysis.
HP-positive subjects were treated with initial eradication therapy, which was consisted of triple therapy before 2012 [23, 24], and 10-day sequential therapy was frequently performed since 2012 [25] as our team found the rapid decrease of eradication rate of triple therapy with an increase of resistance of clarithromycin [23]. The triple therapy regimens consisted of a combination of a standard dose of esomeprazole 40 mg twice per day, amoxicillin 1 g twice per day, and clarithromycin 500 mg twice per day for 1 week [23, 24]. The 10-day sequential therapy includes esomeprazole 40 mg, amoxicillin 1000 mg twice per day for 5 days followed by esomeprazole 40 mg, clarithromycin 500 mg and metronidazole 500 mg twice per day for the next 5 days [25]. The assessment of HP eradication was performed by the 13C-urea breath test (UBiTkit; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) 4 weeks after the eradication therapy (if there was a possibility of false-positive then follow-up gastroscopic biopsy was performed again) or by histology (Giemsa stain) and the rapid urease test when the follow-up gastroscopy was scheduled soon. The patients chose another eradication therapy if the first-line regimen failed: either a 14-day quadruple regimen containing bismuth (esomeprazole 40 mg twice per day, tripotassium dicitrate bismuthate [Denol; Green cross Corp., Yongin, Korea] 300 mg four times per day, metronidazole 500 mg three times per day and tetracycline 500 mg four times per day) or a 14-day moxifloxacin-based triple therapy (moxifloxacin [Avelox; Bayer AG, Leverkusen, Germany] 400 mg daily, esomeprazole 40 mg twice per day, and amoxicillin 1 g twice per day) [24]. The follow-up gastroscopy for GC recurrence was performed every 6 months until 2 years after operation and then every 1 year thereafter.
Statistical analysis
The studied outcomes were overall survival, gastric cancer-specific survival, and gastric cancer recurrence. Cox proportional hazards univariate and multivariate analyses were used for the identification of risk factors, and variables having a P value of less than 0.2 in the univariate analyses were used as covariates for the multivariate analysis. Proportional risk assumptions were verified by observed-expected plot (Supplementary Fig. 2, see online). The Kaplan–Meier estimator method and log-rank tests were used for comparisons of survival. An additional analysis was performed using propensity score matching (PSM) for detailed comparison of the eradicated and non-eradicated groups since there were differences between the two groups. The type of cancer, sex, age, and surgical methods used were matched 1:1 using PSM, and all standard mean differences of each variant were under 0.2. Analyses were performed using IBM SPSS Statistics software (version 25.0; IBM Corp., Armonk, NY, USA). A P value of less than 0.05 was established to indicate the level of significance.
Results
A total of 1,031 patients were enrolled, and 564 (54.7%) patients were not treated for HP. Among 467 patients, 451 (43.7%) patients were successfully eradicated, and the eradication failed in 16 (1.6%) patients (Fig. 1). There was no change in the percentage of eradicated patients over time. Totally 66 patients out of 1031 (6.4%) were found to be recurred, 8 patients in EGC group (8 out of 678, 1.2%) and 58 patients in AGC group (58 out of 353, 16.4%). The incidence of cancer recurrence was higher in the non-eradicated group (56 out of 580, 9.6%) compared to the treatment group (10 out of 451, 2.2%). Regarding the recurrence sites were as following: in the eradicated group (10 cases), recurred sites were peritoneal seeding (including cases which were difficult to verify the exact location of the recurrence such as malignant ascites, multiple abdominal and pelvic cavity lymph node enlargements, or adhesive ileus, 60%), anastomosis site recurrence (30%), bone metastases (10%). In the non-eradicated group, recurred sites were peritoneal seeding (60%), hepatobiliary metastases (25%), anastomosis site recurrence (7%), lung or thorax lymph node enlargement (4%), and brain or leptomeningeal metastases (4%). In both groups, peritoneal seeding was more than a half since most recurred cancer cases were revealed as advanced cancer stage. In the eradicated group, anastomosis site recurrence was the second, while hepatobiliary metastases were the second in non-eradicated group. The number of recurred cases were too small to find statistical significance.
The overall characteristics of the enrolled patients and inter-group differences allocated by HP eradication are shown in Table 1. The median age was 59 years (interquartile range [IQR], 49 to 68 years), and the median follow-up period was 67 months (IQR, 69 to 124 months, maximum 179 months; 306 patients had longer than 10-year follow-up period). Early gastric cancer (EGC) patients were 65.8% and advanced gastric cancer (AGC) patients were 34.2% of overall, which is consistent with previous surveys of Korea [30, 31]. Patients in the eradicated group were statistically younger, had an earlier stage cancer, underwent Billroth I reconstruction compared to the patients in the non-eradicated group (Table 1). However, there was no difference in terms of adjuvant chemotherapy (P = 0.986) (Table 1).
Comparisons of overall survival
In the analyses for OS using the Kaplan–Meier method (Fig. 2a), a survival benefit was observed in the HP-eradicated group compared to the non-eradicated group. The P-value using the log-rank test also reached the level of statistical significance (P < 0.001). To exclude the effect of other possible factors on the OS, PSM was used (Supplementary Fig. 3 and Supplementary Table 1, see online). Analyses after PSM (Fig. 2b) also showed statistical significance (P < 0.001). In addition, a survival benefit was observed in the sub-analysis according to cancer type in the HP-eradicated group compared to the non-eradicated group before and after PSM, with statistical significance [P < 0.001 in both of EGC (Supplementary Fig. 4A) and AGC group (Supplementary Fig. 4B) before PSM; P < 0.001 in both of EGC (Fig. 4a) and AGC group (Fig. 4b) after PSM].
Comparisons of overall survival depending on Helicobacter pylori eradication. The benefits of overall survival were observed in the successfully eradicated group before (a) and after (b) propensity score matching compared to non-eradicated group, with statistical significance (P < 0.001). Cumulative survival was calculated using Kaplan–Meier estimates; the p-value was calculated using the log-rank test
Comparisons of GC-related survival
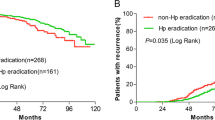
In analyses for GC-related survival using the Kaplan–Meier method (Fig. 3a), a survival benefit was observed in the HP-eradicated group compared to the non-eradicated group. The P-value using the log-rank test reached the level of statistical significance (P < 0.001). Analyses after PSM (Fig. 3b) also showed statistical significance (P < 0.001). In addition, a survival benefit was observed in the sub-analysis according to cancer type in the HP-eradicated group compared to the non-eradicated group before and after PSM, with statistical significance [P = 0.009 in EGC group (Supplementary Fig. 4C), P < 0.001 in AGC group (Supplementary Fig. 4D) before PSM; P = 0.045 in EGC group (Fig. 4c), P = 0.011 in AGC group after PSM (Fig. 4d)].
Comparisons of gastric cancer-specific survival depending on Helicobacter pylori eradication. The benefits of gastric cancer-specific survival were observed in the successfully eradicated group before (a) and after (b) propensity score matching compared to non-eradicated group, with statistical significance (P < 0.001). Cumulative survival was calculated using Kaplan–Meier estimates; the P value was calculated using the log-rank test
Comparisons of overall and gastric cancer-specific survival depending on Helicobacter pylori eradication and cancer type after propensity score matching. Overall survival in early gastric cancer (EGC) (a) and advanced gastric cancer (AGC) (b) patients, gastric cancer-specific survival in EGC (c) and AGC (d) patients. The benefits of gastric cancer-specific survival were observed in the successfully eradicated group compared to non-eradicated group in all cases, with statistical significance. Cumulative survival was calculated using Kaplan–Meier estimates; the P-values were calculated using the log-rank test
Univariate and multivariate analyses for GC-specific mortality and GC recurrence
Results of the univariate and multivariate analyses for GC-specific mortality are shown in Table 2. In the analysis for GC-specific death, age ≥ 60 years, operation method used, histologic type (diffuse type), final cancer stage after surgery, adjuvant chemotherapy, and non-eradicated for HP were associated factors. When multivariate analysis was performed, final cancer stage (stage II, aHR = 9.33, P < 0.001; stage III or above, aHR = 26.17, P < 0.001) and non-eradicated group (aHR = 3.41, P < 0.001) were independent risk factors. Age ≥ 60 years (aHR = 1.52, P = 0.078) and diffuse-type histology were marginally associated with GC-specific death (aHR = 1.57, P = 0.072).
Results of the univariate and multivariate analyses for cancer recurrence are shown in Table 3. In the univariate analysis for GC recurrence, operation method used (Billroth II), histologic type (diffuse type), final cancer stage after surgery, adjuvant chemotherapy, and non-eradicated for HP were associated factors. When multivariate analysis was performed, final cancer stage (stage II, aHR = 7.08, P < 0.001; stage III or above, aHR = 19.64, P < 0.001) and non-eradicated group (aHR = 2.70, P = 0.005) were independent risk factors. Diffuse type histology was marginally associated with GC recurrence (aHR = 1.61, P = 0.076).
Discussion
Our study suggested that HP eradication after subtotal gastrectomy brought the significant benefit of overall- or gastric cancer-specific survival in a large cohort and long-term follow-up study with up to 15 years of follow-up and over 300 patients with follow-up longer than 10 years. In addition, statistically significant survival benefits were observed in sub-group analysis according to cancer types such as EGC and AGC, before and after PSM. In addition, multivariate analysis showed that the final cancer stage and non-eradicated group were independent risk factors. HP positivity as well as final cancer stage were independent risk factors for GC-specific death in Cox proportional hazards univariate and multivariate analyses. Moreover, HP positivity and final cancer stage were independent risk factors for cancer recurrence. As far as we know this is the first report to date showing the survival gain of HP eradication after subtotal gastrectomy not only in EGC but also in AGC.
It is well established that HP plays a crucial role in the occurrence of GC, and HP eradication is now recommended widely in most guidelines for patients who received endoscopic resection for EGC, as HP eradication prevented EGC recurrence and a survival benefit [7, 28, 29]. In addition, the eradication of HP improves the atrophic gastritis and intestinal metaplasia in the general population [30], and in patients treated surgically for GC [10, 11]. In particular, in Japan, which has a high incidence of GC, they have expanded their treatment guidelines and medical insurance coverage to treat HP in all patients who are HP positive, and a benefit in overall survival was reported after active eradication [31,32,33].
On the other hand, patients who underwent surgical resection for GC are reported to have had no favorable results to date regarding on preventing cancer recurrence or improving survival. Thus there have been no set guidelines for HP eradication after surgical treatment of GC. Several studies have reported that patients with an HP-seropositive status had a better prognosis and that HP negativity was an independent prognostic factor for relapse-free survival and overall survival in the analyses of surgically treated patients with GC [3,4,5, 34]. However, in a recent study in Korea, there were no statistically significant differences in long-term survival in a prospective randomized HP eradication trial with 169 patients with GC after distal gastrectomy. The authors pointed out that in previous studies, the HP-negative status group likely included heterogeneous patients who were initially negative for HP infection or those whose HP infection disappeared because of the advances of severe atrophy or HP treatment [35]. Similarly, HP-unassociated GC represents only 2–10% of all GC cases in Japan [36], and the proportion of patients who are truly HP negative was extremely low in regions like Korea, due to the high prevalence of HP [37]. In addition, those previous studies were conducted with relatively small numbers of patients, and the effect of HP eradication was not evaluated.
In the present study, when the effects of the eradication of HP on overall survival, GC-related survival, and recurrence of GC after surgery were analyzed in big cohort with long-term follow-up, the benefits of overall and GC-specific survival were observed in the HP-eradicated group even after PSM. Furthermore, HP positivity was an independent risk factor for GC-specific death and cancer recurrence. There might be several reasons for this. First, HP plays an important role in the recurrence of GC, so HP eradication reduced the recurrence of GC in patients treated with endoscopic resection of GC, naturally leading to improved survival [6, 7]. Second, several recent studies have reported on the systemic effects of HP, such as hypertension and hyperlipidemia. This is supported by a report describing survival improvement after HP eradication in patients with hypertension [38], and an independent role of HP in the pathogenesis of metabolic syndrome [39]. There are other reports about the contribution of HP to arterial stiffness [40], and non-alcoholic fatty liver disease [41]. The decrease in these extra-gastric effects of HP after eradication might contribute to survival improvements. Third, although we matched the baseline clinical characteristics of both groups using PSM, it is still possible that there were differences between the two groups in key details. HP tests and eradication treatments may have been actively conducted in patients who were in relatively good physical condition, who were thought to be able to withstand the side effects of antibiotic treatment such as gastrointestinal disorders, and who had good systemic conditions and an active willingness to treat. Finally, this promising result might be originated from the high proportion of stage I (76.3%) in this GC cohort and because the outcome of GC in Koreans was highest in the world [26]. However, when we performed sub-analysis in the EGC and AGC separately to evaluate this point the benefit of HP eradication looks like to be relatively higher in the AGC than that of EGC. As the 5-year survival rate of EGC patients is over 95% in Korea it could be interpreted that the benefit of HP eradication in AGC patients contributed a difference in whole GC patients in the present study.
This study has a limitation. That is, this study was not a prospectively-designed study, and the analyses were based on patient clinical records from a single institution, rather than a double-blind and randomized control study. However, from the opening of the hospital in 2003, the gastric surgery team at SNUBH made a surgical cohort for GC patients who received surgical treatment. They collected the information on all patients with GC such as the clinical cancer stage, death, recurrence, and medical and social history in the digital system, and the patients were followed-up prospectively by the same protocol and the medical records of these patients are considerably accurate. In addition, about 37% (514 out of 1379) of participated patients were coincided with the pool of medical cohort, in which enrollment was performed by NK at the diagnosis of GC through endoscopy. In spite of this background patients enrolled in this study may have different rates of HP examinations and HP treatment depending on their clinician’s preferences, but NK usually performed the HP tests in the same protocol and HSL (the pathologist) interpreted the HP tests in a consistent manner. To rule out the possible heterogeneity many GC patients with uncertain HP status was excluded in certain criteria. In addition, initial HP eradication was performed by the surgeon with proton pump inhibitor-(PPI)-based triple therapy, but when there was an increase in the failure of HP eradication, they consulted with NK. We analyzed patients who received HP eradication therapy within 2 years after surgery, and patients did not follow this eradication protocol were excluded to avoid the immortal time bias. And considering the possibility of bias that the high rate of EGC affected survival, we confirmed that the proportion of EGC and AGC of the enrolled patients is consistent with previous surveys of Korea [26, 27]. In addition, additional analysis was performed according to the type of GC to confirm that there is a benefit of survival in both EGC and AGC groups. Finally, we also cross-checked the death of patients by comparing the data of the National Statistical Office, and tried additional analyses using PSM, to overcome the limitations and ensure the accuracy. PSM is used to match patients with a similar distribution of confounders so that the difference in outcomes gives an unbiased estimate of treatment effect thereby increasing between-group comparability [42, 43]; therefore, it is increasingly being used in observational studies recently, especially when a randomized control trial is difficult to perform [44, 45].
In conclusion, this study is the first to report a survival improvement after HP eradication in patients treated with surgery on a larger scale and with long-term follow-up data. These results suggest that the indication of future HP treatment guideline could be extended and that intensive screening and treatment for HP should be conducted in patients undergoing surgical treatment of GC regardless of cancer stage.
Change history
09 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10120-023-01403-3
References
Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31.
Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86.
Meimarakis G, Winter H, Assmann I, Kopp R, Lehn N, Kist M, et al. Helicobacter pylori as a prognostic indicator after curative resection of gastric carcinoma: a prospective study. Lancet Oncol. 2006;7:211–22.
Marrelli D, Pedrazzani C, Berardi A, Corso G, Neri A, Garosi L, et al. Negative Helicobacter pylori status is associated with poor prognosis in patients with gastric cancer. Cancer. 2009;115:2071–80.
Kang SY, Han JH, Ahn MS, Lee HW, Jeong SH, Park JS, et al. Helicobacter pylori infection as an independent prognostic factor for locally advanced gastric cancer patients treated with adjuvant chemotherapy after curative resection. Int J Cancer. 2012;130:948–58.
Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–7.
Choi IJ, Kook MC, Kim YI, Cho SJ, Lee JY, Kim CG, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 2018;378:1085–95.
Koulis A, Buckle A, Boussioutas A. Premalignant lesions and gastric cancer: current understanding. World J Gastrointest Oncol. 2019;11:665–78.
Shao L, Li P, Ye J, Chen J, Han Y, Cai J, et al. Risk of gastric cancer among patients with gastric intestinal metaplasia. Int J Cancer. 2018;143:1671–7.
Cho SJ, Choi IJ, Kook MC, Yoon H, Park S, Kim CG, et al. Randomised clinical trial: the effects of Helicobacter pylori eradication on glandular atrophy and intestinal metaplasia after subtotal gastrectomy for gastric cancer. Aliment Pharmacol Ther. 2013;38:477–89.
Hwang JJ, Lee DH, Kang KK, Lee AR, Yoon H, Shin CM, et al. Eradication rate and histological changes after Helicobacter pylori eradication treatment in gastric cancer patients following subtotal gastrectomy. World J Gastroenterol. 2015;21:3936–43.
Yoo S, Lee KH, Lee HJ, Ha K, Lim C, Chin HJ, et al. Seoul national university bundang hospital's electronic system for total care. Healthc Inform Res. 2012;18:145–52.
Bae S, Kim N, Kang JM, Kim DS, Kim KM, Cho YK, et al. Incidence and 30-day mortality of peptic ulcer bleeding in Korea. Eur J Gastroenterol Hepatol. 2012;24:675–82.
Bae S, Shim KN, Kim N, Kang JM, Kim DS, Kim KM, et al. Incidence and short-term mortality from perforated peptic ulcer in Korea: a population-based study. J Epidemiol. 2012;22:508–16.
Hwang YJ, Kim N, Yun CY, Yoon H, Shin CM, Park YS, et al. Validation of administrative big database for colorectal cancer searched by international classification of disease 10th codes in Korean: a retrospective big-cohort Study. J Cancer Prev. 2018;23:183–90.
Hwang YJ, Park SM, Ahn S, Lee JC, Park YS, Kim N. Accuracy of an administrative database for pancreatic cancer by international classification of disease 10th codes: a retrospective large-cohort study. World J Gastroenterol. 2019;25:5619–29.
Hwang YJ, Park SM, Ahn S, et al. Diagnostic accuracy of administrative database for bile duct cancer by ICD-10 code in a tertiary institute in Korea. Hepatobiliary Pancreat Dis Int. 2020. https://doi.org/10.1016/j.hbpd.2020.03.002.
Yun CY, Kim N, Lee J, Lee JY, Hwang YJ, Lee HS, et al. Usefulness of OLGA and OLGIM system not only for intestinal type but also for diffuse type of gastric cancer, and no interaction among the gastric cancer risk factors. Helicobacter. 2018;23:e12542.
Baek SM, Kim N, Kwon YJ, Lee HS, Kim HY, Lee J, et al. Role of serum pepsinogen II and Helicobacter pylori status in the detection of diffuse-type early gastric cancer in young individuals in South Korea. Gut Liver. 2020. https://doi.org/10.5009/gnl19091.
Yoon K, Kim N, Kim J, Lee JW, Lee HS, Lee JC, et al. Dynamic changes in Helicobacter pylori status following gastric cancer surgery. Gut Liver. 2017;11:209–15.
Kim HJ, Kwon M, Kim N, Lee JB, Won S. The influence of family history on stage and survival of gastric cancer according to the TGFB1 C-509T polymorphism in Korea. Gut Liver. 2020;14:79–88.
Lopes AI, Vale FF, Oleastro M. Helicobacter pylori infection - recent developments in diagnosis. World J Gastroenterol. 2014;20:9299–313.
Lee JY, Kim N, Kim MS, Choi YJ, Lee JW, Yoon H, et al. Factors affecting first-line triple therapy of Helicobacter pylori including CYP2C19 genotype and antibiotic resistance. Dig Dis Sci. 2014;59:1235–43.
Yoon K, Kim N, Nam RH, Suh JH, Lee S, Kim JM, et al. Ultimate eradication rate of Helicobacter pylori after first, second, or third-line therapy in Korea. J Gastroenterol Hepatol. 2015;30:490–5.
Yoon K, Kim N. Eradication rates of 10-day sequential therapy for Helicobacter pylori: Results of an 8-year prospective study conducted at a tertiary Korean hospital. Korean J Gastroenterol. 2019;73:99–104.
Information Committee of Korean Gastric Cancer Association. Korean gastric cancer association nationwide survey on gastric cancer in 2014. J Gastric Cancer. 2016;16:131–40.
Kim JH, Kim SS, Lee JH, Jung DH, Cheung DY, Chung WC, et al. Early detection is important to reduce the economic burden of gastric cancer. J Gastric cancer. 2018;18:82–9.
Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–39.
Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017;66:6–30.
Hwang YJ, Kim N, Lee HS, Lee JB, Choi YJ, Yoon H, et al. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication - a prospective study for up to 10 years. Aliment Pharmacol Ther. 2018;47:380–90.
Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–67.
Hiroi S, Sugano K, Tanaka S, Kawakami K. Impact of health insurance coverage for Helicobacter pylori gastritis on the trends in eradication therapy in Japan: retrospective observational study and simulation study based on real-world data. BMJ Open. 2017;7:e015855.
Uno Y. Prevention of gastric cancer by Helicobacter pylori eradication: a review from Japan. Cancer Med. 2019;8:3992–4000.
Lee WJ, Lin JT, Shun CT, Lee WC, Yu SC, Lee PH, et al. Comparison between resectable gastric adenocarcinomas seropositive and seronegative for Helicobacter pylori. Br J Surg. 1995;82:802–5.
Kim YI, Cho SJ, Lee JY, Kim CG, Kook MC, Ryu KW, et al. Effect of Helicobacter pylori Eradication on long-term survival after distal gastrectomy for gastric cancer. Cancer Res Treat. 2016;48:1020–9.
Kato S, Matsukura N, Tsukada K, Matsuda N, Mizoshita T, Tsukamoto T, et al. Helicobacter pylori infection-negative gastric cancer in Japanese hospital patients: incidence and pathological characteristics. Cancer Sci. 2007;98:790–4.
Yoon H, Kim N, Lee HS, Shin CM, Park YS, Lee DH, et al. Helicobacter pylori-negative gastric cancer in South Korea: incidence and clinicopathologic characteristics. Helicobacter. 2011;16:382–8.
Kim YI, Kim YA, Lee JW, Kim HJ, Kim SH, Kim SG, et al. Effect of Helicobacter pylori treatment on long-term mortality in patients with hypertension. Gut Liver. 2020;14:47–56.
Lim SH, Kim N, Kwon JW, Kim SE, Baik GH, Lee JY, et al. Positive association between Helicobacter pylori infection and metabolic syndrome in a Korean population: a multicenter nationwide study. Dig Dis Sci. 2019;64:2219–30.
Choi JM, Lim SH, Han YM, Lee H, Seo JY, Park HE, et al. Association between Helicobacter pylori infection and arterial stiffness: results from a large cross-sectional study. PLoS ONE. 2019;14:e0221643.
Chen C, Zhang C, Wang X, Zhang F, Zhang Z, Ma P, Feng S. Helicobacter pylori infection may increase the severity of nonalcoholic fatty liver disease via promoting liver function damage, glycometabolism, lipid metabolism, inflammatory reaction and metabolic syndrome. Eur J Gastroenterol Hepatol. 2019. https://doi.org/10.1097/MEG.0000000000001601.
Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53:1112–7.
Baek S, Park SH, Won E, Park YR, Kim HJ. Propensity score matching: a conceptual review for radiology researchers. Korean J Radiol. 2015;16:286–96.
Lee SW, Kwon JH, Lee HL, Yoo SH, Nam HC, Sung PS, et al. Comparison of tenofovir and entecavir on the risk of hepatocellular carcinoma and mortality in treatment-naïve patients with chronic hepatitis B in Korea: a large-scale, propensity score analysis. Gut. 2019. https://doi.org/10.1136/gutjnl-2019-318947.
Guo CG, Cheung KS, Zhang F, Chan EW, Chen L, Wong IC, Leung WK. Incidences, temporal trends and risks of hospitalisation for gastrointestinal bleeding in new or chronic low-dose aspirin users after treatment for Helicobacter pylori: a territory-wide cohort study. Gut. 2020;69:445–52.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant for the Global Core Research Center (GCRC), funded by the Korean government (MSIP) (No. 2011-0030001).
Author information
Authors and Affiliations
Contributions
YC analyzed the data, provided statistical support, and drafted the article; NK designed this study, collected the data and edited the manuscript; CYY checked the survival rate of patients with gastric cancer; YJC, HY, CMS and YSP performed endoscopies for the diagnosis of gastric cancer and edited the text; SHA, DJP and HHK performed surgeries for gastric cancer; HSL performed the histologic diagnosis of gastric cancer and the histologic identification of Helicobacter pylori; J-WK, JWK and K-WL performed chemotherapy in case of advanced gastric cancer; WC, JHP, YJL, KHL and Young Hoon Kim performed the radiologic study; DHL advised on the design of this study and supervised manuscript preparation; HHK kindly provided surgical cohort information and supervised manuscript preparation. All authors have read and approved the final draft of this paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest relevant to this article to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guarantor of the article: Professor Nayoung Kim.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, Y., Kim, N., Yun, C.Y. et al. Effect of Helicobacter pylori eradication after subtotal gastrectomy on the survival rate of patients with gastric cancer: follow-up for up to 15 years. Gastric Cancer 23, 1051–1063 (2020). https://doi.org/10.1007/s10120-020-01076-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-020-01076-2