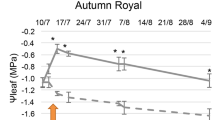
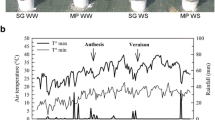
Abstract
Grapevine is an extremely important crop worldwide. In southern Europe, post-flowering phases of the growth cycle can occur under high temperatures, excessive light, and drought conditions at soil and/or atmospheric level. In this study, we subjected greenhouse grown grapevine, variety Aragonez, to two individual abiotic stresses, water deficit stress (WDS), and heat stress (HS). The adaptation of plants to stress is a complex response triggered by cascades of molecular networks involved in stress perception, signal transduction, and the expression of specific stress-related genes and metabolites. Approaches such as array-based transcript profiling allow assessing the expression of thousands of genes in control and stress tissues. Using microarrays, we analyzed the leaf transcriptomic profile of the grapevine plants. Photosynthesis measurements verified that the plants were significantly affected by the stresses applied. Leaf gene expression was obtained using a high-throughput transcriptomic grapevine array, the 23K custom-made Affymetrix Vitis GeneChip. We identified 1,594 genes as differentially expressed between control and treatments and grouped them into ten major functional categories using MapMan software. The transcriptome of Aragonez was more significantly affected by HS when compared with WDS. The number of genes coding for heat-shock proteins and transcription factors expressed solely in response to HS suggesting their expression as unique signatures of HS. However, a cross-talk between the response pathways to both stresses was observed at the level of AP2/ERF transcription factors.








Similar content being viewed by others
References
Adam Z, Adamska I, Nakabayashi K et al (2001) Chloroplast and mitochondrial proteases in Arabidopsis. A proposed nomenclature. Plant Physiol 125:1912–1918
Chaves MM, Santos T, Souza CR et al (2007) Deficit irrigation in grapevine improves water-use efficiency while controlling vigour and production quality. Ann Appl Biol 150:237–252
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Chaves M, Zarrouk O, Francisco R et al (2010) Grapevine under deficit irrigation: hints from physiological and molecular data. Ann Bot 105(5):661–676
Chen W, Provart NJ, Glazebrook J et al (2002) Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell 14:559–574
Chen H, Lai Z, Shi J et al (2010) Roles of Arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biol 10:281
Coito JL, Rocheta M, Carvalho LC et al (2012) Microarray-based uncovering reference genes for quantitative real time PCR in grapevine under abiotic stress. BMC Res Notes 5:220
Cramer GC (2010) Abiotic stress and plant responses from the whole vine to the genes. Aust J Grape Wine Res 16:89–93
Cramer GC, Ergül A, Grimplet J et al (2007) Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Funct Integr Genomics 7:111–134
Cui X, Loraine AE (2009) Consistency analysis of redundant probe sets on Affymetrix three-prime expression arrays and applications to differential mRNA processing. PLoS ONE 4(1):e4229
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282
Gadjev I, Vanderauwera S, Gechev TS et al (2006) Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol 141:436–445
Ganea E (2001) Chaperone-like activity of alpha-crystallin and other small heat shock proteins. Curr Protein Pept Sci 2:205–225
Grimplet J, Cramer G, Dickerson J et al (2009) VitisNet: “Omics” integration through grapevine molecular networks. PLoS ONE 4:e8365
Gupta SC, Sharma A, Mishra M et al (2010) Heat shock proteins in toxicology: how close and how far? Life Sci 86:377–384
Hamm HE (1998) The many faces of G protein signaling. J Biol Chem 273:669–672
Iba K (2002) Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol 53:225–245
Jones GV, White MA, Owen RC et al (2005) Climate change and global wine quality. Clim Change 73:319–343
Khanna-Chopra R, Srivalli B, Ahlawat YS (1999) Drought induces many forms of cysteine proteases not observed during natural senescence. Biochem Biophys Res Commun 255:324–327
Knight H (2000) Calcium signaling during abiotic stress in plants. Int Rev Cytol 195:269–324
Knight H, Knight MR (2001) Abiotic stress signalling pathways: specificity and cross-talk. Trends Plant Sci 6:262–267
Koskull-Döring P, Scharf K-D, Nover L (2007) The diversity of plant heat stress factors. Trends Plant Sci 12:452–457
Kotak S, Larkindale J, Lee U et al (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10:310–316
Larkindale J, Vierling E (2008) Core genome responses involved in acclimation to high temperature. Plant Physiol 146:748–761
Larkindale J, Hall JD, Knight MR et al (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 138:882–897
Li C, Wong WH (2001a) Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A 98:31–36
Li C, Wong WH (2001b) Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol 2(8):1–11
Liang M, Haroldsen V, Cai X et al (2006) Expression of a putative laccase gene, ZmLAC1, in maize primary roots under stress. Plant Cell Environ 29:746–753
Liu Q, Kasuga M, Sakuma Y et al (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
Mayer MP, Bukau B (1999) Molecular chaperones: the busy life of Hsp90. Curr Biol 9:R322–R325
Mazzucotelli E, Mastrangelo AM, Crosatti C et al (2008) Abiotic stress response in plants: when post-transcriptional and post-translational regulations control transcription. Plant Sci 174:420–431
Medrano H, Escalona JM, Cifre J et al (2003) A ten-year study on the physiology of two Spanish grapevine cultivars under field conditions: effects of water availability from leaf photosynthesis to grape yield and quality. Funct Plant Biol 30:607–619
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19
Nover L, Scharf KD, Gagliardi D et al (1996) The Hsf world: classification and properties of plant heat stress transcription factors. Cell Stress Chaperon 1:215–223
Pontin M, Piccoli P, Francisco R et al (2010) Transcriptome changes in grapevine (Vitis vinifera L.) cv. Malbec leaves induced by ultraviolet-B radiation. BMC Plant Biol 10:224
Reid KE, Olsson N, Schlosser J et al (2006) An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol 6:27
Reusink WH, Buell CR (2005) Microarray expression profiling resources for plant genomics. Trends Plant Sci 10:603–609
Rhue RD, Grogan CO, Stockmeyer EW et al (1978) Genetic control of aluminium tolerance in corn. Crop Sci 18:1063–1067
Riechmann JL, Heard J, Martin G et al (2000) Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290:2105–2110
Rizhsky L, Hongjian L, Shuman J et al (2004) When defense pathways collide: the response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134:1–14
Roy SJ, Tucker EJ, Tester M (2011) Genetic analysis of abiotic stress tolerance in crops. Curr Opin Plant Biol 14:232–239
Rushton PJ, Somssich IE, Ringler P et al (2010) WRKY transcription factors. Trends Plant Sci 15:247–258
Sangster TA, Queitsch C (2005) The HSP90 chaperone complex, an emerging force in plant development and phenotypic plasticity. Curr Opin Plant Biol 8:86–92
Santner A, Estelle M (2010) The ubiquitin–proteasome system regulates plant hormone signaling. Plant J 61:1029–1040
Siddique M, Gernhard S, Koskull-Döring P et al (2008) The plant sHSP superfamily: five new members in Arabidopsis thaliana with unexpected properties. Cell Stress Chaperon 13:183–197
Simova-Stoilova L, Vaseva I, Grigorova B et al (2010) Proteolytic activity and cysteine protease expression in wheat leaves under severe soil drought and recovery. Plant Physiol Bioch 48:200–206
Sousa TA, Oliveira MT, Pereira JM (2006) Physiological indicators of plant water status of irrigated and non-irrigated grapevines in low rainfall area of Portugal. Plant Soil 282:127–134
Sreenivasulu N, Sopory SK, Kishor PBK (2007) Deciphering the regulatory mechanisms of abiotic stress tolerance in plants by genomic approaches. Gene 388:1–13
Swindell WR, Huebner M, Weber AP (2007) Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genomics 8:125
Tattersall EA, Grimplet J, DeLuc L et al (2007) Transcript abundance profiles reveal larger and more complex responses of grapevine to chilling compared to osmotic and salinity stress. Funct Integr Genomics 7(4):317–33
Thimm O, Blasing O, Gibon Y et al (2004) MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37(6):914–939
Umezawa T, Fujita M, Fujita Y et al (2006) Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Curr Opin Biotechnol 17:113–122
Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Op Biotech 16:123–132
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures. Towards genetic engineering for stress tolerance. Planta 218:1–14
Wang L-J, Fan L, Loescher W et al (2010) Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol 10:34
Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the coordination of responses to stress in plants. Plant Cell Environ 25:195–210
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance do dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Yang X, Kalluri UC, Jawdy S et al (2008) The F-Box gene family is expanded in herbaceous annual plants relative to woody perennial plants. Plant Physiol 148:1189–1200
Acknowledgments
The authors would like to thank Jose Miguel Zapater, Gerôme Grimplet, and Pablo Carbonelle for GrapeGen GeneChip 12Xv0 annotations and MapMan files. The research was funded by Fundação para a Ciência e Tecnologia: project PTDC/AGR-GPL/099624/2008; CBAA (PestOE/AGR/UI0240/2011); ERA-NET Plant Genomics 006/2006; and the post-doc grant SFRH/BPD/64905/2009 to MR.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Light response (A/I) curves measured on fully expanded leaves of control plants and plants subjected to HS and WDS (DOCX 64 kb)
Supplementary Fig. 2
Comparison of gene expression ratios obtained by microarray and by qRT-PCR. Expression profiles are shown for randomly chosen transcripts whose expression was significantly up- or down-regulated in the microarray analysis in HS and in WDS. The microarray fold change are plotted on the Y-axis against the log2(expression ratio) values obtained by qRT-PCR on the X-axis. The scales of the X- and Y-axes are different, for clarity purposes (DOCX 40 kb)
Supplementary Fig. 3
List of genes differentially expressed under water stress. Complete list of the genes differentially expressed in WDS including Probe-set ID, Unique grapevine gene ID, Functional Categories according to GrapeGen annotation and Fold-Change (log2) (XLSX 324 kb)
Supplementary Fig. 4
List of genes differentially expressed under heat stress. XLS gene files showing a complete list of the genes differentially expressed in HS including Probe-set ID, Unique grapevine gene ID, Functional Categories according to GrapeGen annotation and Fold-Change (log2) (XLSX 310 kb)
Supplementary Fig. 5
Representative of GCC-box transcription factors binding domains present in promoter genes commonly expressed in WDS and HS with log2 fold change higher than three in at least one experimental condition (Supplementary Fig. 10). Binding sites were annotated through PLACE (http://www.dna.affrc.go.jp/PLACE/index.html) (Higo et al. 1999) and Prosite (http://prosite.expasy.org/) (Sigrist et al. 2012) along 1,000 base pair upstream of the target gene (arrow in red). (PDF 136 kb)
Supplementary Fig. 6
Protein metabolism and modification genes differentially expressed under water and heat stress. XLS genes file showing a list of the genes differentially expressed in both stresses according to MapMan pictorial representation (Fig. 4) including Bin code, Bin name, Gene Unique ID, Fold-Change (log2), NCBI Accession and Putative Function (XLSX 22 kb)
Supplementary Fig. 7
Transcription factors differentially expressed under water and heat stress. XLS genes file showing a list of the TFs differentially expressed in both stresses according to MapMan pictorial representation (Fig. 4) including Bin code, Bin name, Gene Unique ID, Fold-Change (log2), NCBI Accession and Putative Function (XLSX 18 kb)
Supplementary Fig. 8
Abiotic stress genes differentially expressed under water and heat stress. XLS genes file showing a list of the abiotic stress responsive genes differentially expressed in both stresses according to MapMan pictorial representation (Fig. 6) including Bin code, Bin name, Gene Unique ID, Fold-Change (log2), NCBI Accession and Putative Function (XLSX 14 kb)
Supplementary Fig. 9
Signalling genes differentially expressed under water and heat stress. XLS genes file showing a list of the signalling genes differentially expressed in both stresses according to MapMan pictorial representation (Fig. 7) including Bin code, Bin name, Gene Unique ID, Fold-Change (log2), NCBI Accession and Putative Function (XLSX 30 kb)
Supplementary Fig. 10
Genes that are differentially expressed in both water and heat stresses. XLS file showing a list of all the genes differentially expressed in both stresses according to MapMan pictorial representation including Bin code, Bin name, Gene Unique ID, Fold-Change (log2), NCBI Accession and Putative Function (XLSX 14 kb)
Rights and permissions
About this article
Cite this article
Rocheta, M., Becker, J.D., Coito, J.L. et al. Heat and water stress induce unique transcriptional signatures of heat-shock proteins and transcription factors in grapevine. Funct Integr Genomics 14, 135–148 (2014). https://doi.org/10.1007/s10142-013-0338-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-013-0338-z