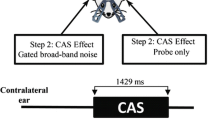
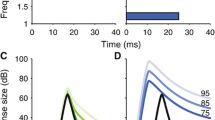
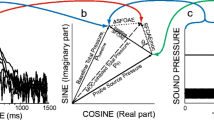
Abstract
The response of the inner ear is modulated by the middle ear muscle (MEM) and olivocochlear (OC) efferent systems. Both systems can be activated reflexively by acoustic stimuli delivered to one or both ears. The acoustic middle ear muscle reflex (MEMR) controls the transmission of acoustic signals through the middle ear, while reflex activation of the medial component of the olivocochlear system (the MOCR) modulates cochlear mechanics. The relative prominence of the two efferent systems varies widely between species. Measuring the effect of either of these systems can be confounded by simultaneously activating the other. We describe a simple, sensitive online method that can identify the effects both systems have on otoacoustic emissions (OAEs) evoked by transient stimuli such as clicks or tone pips (TEOAEs). The method detects directly in the time domain the changes in the stimulus and/or emission pressures caused by contralateral noise. Measurements in human participants are consistent with other reports that the threshold for MOCR activation is consistently lower than for MEMR. The method appears to control for drift and subject-generated noise well enough to avoid the need for post hoc processing, making it promising for application in animal experiments (even if awake) and in the hearing clinic.








Similar content being viewed by others
Notes
The response to an additional set of 4 probe and 1 reference pips was not included in the averaging in case of onset artifacts. As none were detected, this step was probably not necessary.
Subsequent to collecting data for this study, the averaging algorithm was modified to create the “signal” and “noise” buffers (A and B) using the results from individual stimulus blocks into alternating A and B “accumulator buffers” rather than separate sequential averages (for A and then B). This is likely to have further reduced the influence of slow drift.
References
Backus BC (2007) Bias due to noise in otoacoustic emissions measurements. J Acoust Soc Am 121:1588–1603
Backus BC, Guinan JJ (2006) Time-course of the human medial olivocochlear reflex. J Acoust Soc Am 119:2889–2904
Backus BC, Guinan JJ (2007) Measurement of the distribution of medial olivocochlear acoustic reflex strengths across normal-hearing individuals via otoacoustic emissions. J Assoc Res Otolaryngol 8:484–496
Boothalingam S, Purcell DW (2015) Influence of the stimulus presentation rate on medial olivocochlear system assays. J Acoust Soc Am 137:724–732
Borg E (1982) Time course of the human acoustic stapedius reflex: a comparison of eight different measures in normal-hearing subjects. Scand Audiol 11:237–242
Charaziak KK, Siegel JH (2015) Tuning of SFOAEs evoked by low-frequency tones is not compatible with localized emission generation. J Assoc Res Otolaryngol 16:317–329
Collet L, Kemp DT, Veuillet E, Duclaux R, Moulin A, Morgon A (1990) Effect of contralateral auditory stimuli on active cochlear micromechanical properties in human subjects. Hear Res 43:251–261
Cooper NP, Guinan JJ (2003) Separate mechanical processes underlie fast and slow effects of medial olivocochlear efferent activity. J Phsyiol 548:307–312
Deeter R, Abel R, Calandruccio L, Dhar S (2009) Contralateral acoustic stimulation alters the magnitude and phase of distortion product otoacoustic emissions. J Acoust Soc Am 126:2413–2424
Delano PH, Elgueda D, Hamame CM, Robles L (2007) Selective attention to visual stimuli reduces cochlear sensitivity in chinchillas. J Neurosci 27:4146–4153
Dolan DF, Guo MH, Nuttall AL (1997) Frequency-dependent enhancement of basilar membrane velocity during olivocochlear bundle stimulation. J Acoust Soc Am 102:3587–3596
Feeney MP, Keefe DH, Marryott LP (2003) Contralateral acoustic reflex thresholds for tonal activators using wideband energy reflectance and admittance. J Speech Lang Hear Res 46:128–136
Feeney MP, Keefe DH, Hunter, LL, Fitzpatrick DF, Garinis AC, Putterman DB, McMillan GP (2017) Normative wideband reflectance, equivalent admittance at the tympanic membrane, and acoustic stapedius reflex threshold in adults. Ear Hear 38. doi:10.1097/AUD.0000000000000399
Goodman SS, Keefe DH (2006) Simultaneous measurement of noise-activated middle-ear muscle reflex and stimulus frequency otoacoustic emissions. J Assoc Res Otolaryngol 7:125–139
Goodman SS, Fitzpatrick DF, Ellison JC, Jesteadt W, Keefe DH (2009) High-frequency click-evoked otoacoustic emissions and behavioral thresholds in humans. J Acoust Soc Am 125:1014–1032
Goodman SS, Mertes IB, Lewis JD, Weissbeck DK (2013) Medial olivocochlear-induced transient-evoked otoacoustic emission amplitude shifts in individual subjects. J Assoc Res Otolaryngol 14:829–842
Gorga MP, Neely ST, Bergman BM, Beauchaine KL, Kaminski JR, Peters J, Schulte L, Jesteadt W (1993) A comparison of transient-evoked and distortion product otoacoustic emissions in normal-hearing and hearing-impaired subjects. J Acoust Soc Am 94:2639–2648
Guinan JJ (2006) Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear 27:589–607
Guinan JJ (2010) Cochlear efferent innervation and function. Curr Opin Otolaryngol Head Neck Surg 18:447–453
Guinan JJ Jr, Backus BC, Lilaonitkul W, Aharonson V (2003) Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J Assoc Res Otolaryngol 4:521–540
Henin S, Long GR, Thompson S (2014) Wideband detection of middle ear muscle activation using swept-tone distortion product otoacoustic emissions. J Acoust Soc Am 136:272–283
Hood LJ, Berlin CI, Bordelon J, Rose K (2003) Patients with auditory neuropathy/dys-synchrony lack efferent suppression of transient evoked otoacoustic emissions. J Am Acad Audiol 14:302–313
Hood LJ, Berlin CI, Hurley A, Cecola RP, Bell B (1996) Contralateral suppression of transient-evoked otoacoustic emissions in humans: intensity effects. Hear Res 101:113–118
Kalluri R, Shera CA (2007) Comparing stimulus-frequency otoacoustic emissions measured by compression, suppression, and spectral smoothing. J Acoust Soc Am 122:3562–3575
Kalluri R, Shera CA (2013) Measuring stimulus-frequency otoacoustic emissions using swept tones. J Acoust Soc Am 134:356–368
Keefe DH, Fitzpatrick D, Liu Y-W, Sanford CA, Gorga MP (2010) Wideband acoustic-reflex test in a test battery to predict middle ear dysfunction. Hear Res 263:52–65
Keefe DH, Feeney MP, Hunter LL, Fitzpatrick DF (2016) Comparisons of transient evoked otoacoustic emissions using chirp and click stimuli. J Acoust Soc Am 140:1949–1973
Keefe DH, Feeney MP, Hunter LL, Fitzpatrick DF (2017) Aural acoustic stapedius-muscle reflex threshold procedures to test human infants and adults. J Assoc Res Otolaryngol 18:65–88
Kemp DT, Chum R (1980) Properties of the generator of stimulated acoustic emissions. Hear Res 2:213–232
Kemp DT, Bray P, Alexander L, Brown AM (1986) Acoustic emission cochleography—practical aspects. Scand Audiol Suppl 25:71–95
Kemp DT, Ryan S, Bray P (1990) A guide to the effective use of otoacoustic emissions. Ear Hear 11:93–105
Lee J, Dhar S, Abel R, Banakis R, Grolley E, Lee J, Zecker S, Siegel JH (2012) Behavioral hearing thresholds between 0.125 and 20 kHz using depth-compensated ear simulator calibration. Ear Hear 33:315–329
Liberman MC, Puria S, Guinan JJ Jr (1996) The ipsilaterally evoked olivocochlear reflex causes rapid adaptation of the 2 f 1 –f 2 distortion product otoacoustic emission. J Acoust Soc Am 99:3572–3583
Liberman MC, Liberman LD, Maison SF (2014) Efferent feedback slows cochlear aging. J Neurosci 34:4599–4607
Maison SF, Liberman MC (2000) Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci 20:4701–4707
Maison SF, Usubuchi H, Liberman MC (2013) Efferent feedback minimizes cochlear neuropathy from moderate noise exposure. J Neurosci 33:5542–5552
Marks K, Siegel JH (2011) Separating olivocochlear and acoustic reflex actions on otoacoustic emissions in the time domain. Assoc Res Otolaryngol Abst 34:125
Marshall L, Lapsley Miller JA, Guinan JJ, Shera CA, Reed CM, Perez ZD, Delhorne LA, Boege P (2014) Otoacoustic-emission-based medial-olivocochlear reflex assays for humans. J Acoust Soc Am 136:2697–2713
Mertes IB, Goodman SS (2016) Within- and across-subject variability of repeated measurements of medial olivocochlear-induced changes in transient-evoked otoacoustic emissions. Ear Hear 37:e72–e84
Moleti A, Botti T, Sisto R (2012) Transient-evoked otoacoustic emission generators in a nonlinear cochlea. J Acoust Soc Am 131:2891–2903
Moleti A, Sisto R, Lucertini M (2014) Experimental evidence for the basal generation place of the short-latency transient-evoked otoacoustic emissions. J Acoust Soc Am 135:2862–2872
Møller AR (2012) Acoustic middle ear reflex. In: Hearing anatomy, physiology, and disorders of the auditory system. Plural Publ, San Diego, pp 241–256
Mountain DC (1980) Changes in endolymphatic potential and crossed olivocochlear bundle stimulation alter cochlear mechanics. Science 210:71–72
Murugasu E, Russell IJ (1996) The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J Neurosci 16:325–332
Neely ST, Liu Z (1994) EMAV: otoacoustic emission averager. Technical Memo No. 17. Boys Town National Research Hospital, Omaha
Neely ST, Stevenson R (2002) SysRes. Technical Memo No. 19. Boys Town National Research Hospital, Omaha
Rasetshwane DM, Neely ST (2011) Calibration of otoacoustic emission probe microphones. J Acoust Soc Am 130:EL238
Relkin EM, Sterns A, Azeredo W, Prieve BA, Woods CI (2005) Physiological mechanisms of onset adaptation and contralateral suppression of DPOAEs in the rat. J Assoc Res Otolaryngol 6:119–135
Robles L, Delano PH (2008) Efferent system. In: Dallos P, Oertel D (eds) The senses: a comprehensive reference. Academic, London, pp 413–445
Shera CA, Zweig G (1993) Noninvasive measurement of the cochlear traveling-wave ratio. J Acoust Soc Am 93:3333–3352
Siegel JH (2002) Calibrating otoacoustic emission probes. In: Robinette MS, Glattke TJ (eds) Otoacoustic emissions: clinical applications, 3rd edn. Thieme Medical, New York, pp 416–441
Siegel JH, Kim DO (1982) Efferent neural control of cochlear mechanics? Olivocochlear bundle stimulation affects cochlear biomechanical nonlinearity. Hear Res 6:171–182
Siegel JH, Charaziak K, Cheatham MA (2011) Transient- and tone-evoked otoacoustic emissions in three species. AIP Conf Proc 1403:307–314
Sisto R, Moleti A, Shera CA (2015) On the spatial distribution of the reflection sources of different latency components of otoacoustic emissions. J Acoust Soc Am 137:768–776
Songer JE, Rosowski JJ (2005) The effect of superior canal dehiscence on cochlear potential in response to air-conducted stimuli in chinchilla. Hear Res 210:53–62
Souza N, Dhar S, Neely ST, Siegel JH (2014) Comparison of nine methods to estimate ear-canal stimulus levels. J Acoust Soc Am 136:1768–1787
Valero MD, Hancock KE, Liberman MC (2016) The middle ear muscle reflex in the diagnosis of cochlear neuropathy. Hear Res 332:29–38
Whitehead ML, Martin GK, Lonsbury-Martin BL (1991) Effects of the crossed acoustic reflex on distortion-product optoacoustic emissions in awake rabbits. Hear Res 51:55–72
Winslow RL, Sachs MB (1987) Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tones in noise. J Neurophysiol 57:1002–1021
Wolter NE, Harrison RV, James JL (2014) Separating the contributions of olivocochlear and middle ear muscle reflexes in modulation of distortion product otoacoustic emission levels. Audiol Neurotol 19:41–48
Xu Y, Cheatham MA, Siegel JH (2015) Separating medial olivocochlear from acoustic reflex effects on transient evoked otoacoustic emissions in unanesthetized mice. In: Proceedings of the 12th international mechanics of hearing workshop. Date: 23–29 June 2014 Location: Cape Sounio, Greece. Edited by DP Corey and KD Karavitaki. AIP conf. proc. 1703:090026
Xu Y, Cheatham MA, Siegel JH (2017) Identifying the origin of effects of contralateral noise on transient evoked otoacoustic emissions in unanesthetized mice. J Assoc Res Otolaryngol. doi:10.1007/s10162-017-0616-x
Zhao W, Dhar S (2010) The effect of contralateral acoustic stimulation on spontaneous otoacoustic emissions. J Assoc Res Otolaryngol 11:53–67
Zhao W, Dhar S (2011) Fast and slow effects of medial olivocochlear efferent activity in humans. PLoS One 6:e18725
Acknowledgements
This study was funded by the Hugh Knowles Hearing Center and Northwestern University. We are very grateful for the thorough and helpful feedback from three anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Marks, K.L., Siegel, J.H. Differentiating Middle Ear and Medial Olivocochlear Effects on Transient-Evoked Otoacoustic Emissions. JARO 18, 529–542 (2017). https://doi.org/10.1007/s10162-017-0621-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-017-0621-0