Abstract
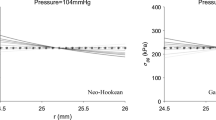
It is now a rather common approach to perform patient-specific stress analyses of arterial walls using finite-element models reconstructed from gated medical images. However, this requires to compute for every Gauss point the deformation gradient between the current configuration and a stress-free reference configuration. It is technically difficult to define such a reference configuration, and there is actually no guarantee that a stress-free configuration is physically attainable due to the presence of internal stresses in unloaded soft tissues. An alternative framework was proposed by Bellini et al. (Ann Biomed Eng 42(3):488–502, 2014). It consists of computing the deformation gradients between the current configuration and a prestressed reference configuration. We present here the first finite-element results based on this concept using the Abaqus software. The reference configuration is set arbitrarily to the in vivo average geometry of the artery, which is obtained from gated medical images and is assumed to be mechanobiologically homeostatic. For every Gauss point, the stress is split additively into the contributions of each individual load-bearing constituent of the tissue, namely elastin, collagen, smooth muscle cells. Each constituent is assigned an independent prestretch in the reference configuration, named the deposition stretch. The outstanding advantage of the present approach is that it simultaneously computes the in situ stresses existing in the reference configuration and predicts the residual stresses that occur after removing the different loadings applied onto the artery (pressure and axial load). As a proof of concept, we applied it on an ideal thick-wall cylinder and showed that the obtained results were consistent with corresponding experimental and analytical results of the well-known literature. In addition, we developed a patient-specific model of a human ascending thoracic aneurysmal aorta and demonstrated the utility in predicting the wall stress distribution in vivo under the effects of physiological pressure. Finally, we simulated the whole process preceding traditional in vitro uniaxial tensile testing of arteries, including excision from the body, radial cutting, flattening and subsequent tensile loading, showing how this process may impact the final mechanical properties derived from these in vitro tests.










Similar content being viewed by others
References
Beller CJ, Labrosse MR, Thubrikar MJ, Robicsek F (2004) Role of aortic root motion in the pathogenesis of aortic dissection. Circulation 109(6):763–769
Bellini C, Ferruzzi J, Roccabianca S, Di Martino ES, Humphrey JD (2014) A microstructurally motivated model of arterial wall mechanics with mechanobiological implications. Ann Biomed Eng 42(3):488–502
Bellini C, Kristofik NJ, Bersi MR, Kyriakides TR Humphrey JD (2017) A hidden structural vulnerability in the thrombospondin-2 deficient aorta increases the propensity to intramural delamination. J Mech Behav Biomed Mater 71:397–406
Bersi MR, Bellini C, Di Achille P, Humphrey JD, Genovese K, Avril S (2016) Novel methodology for characterizing regional variations in the material properties of murine aortas. J Biomech Eng. doi:10.1115/1.4033674
Cardamone L, Valentín A, Eberth JF, Humphrey JD (2009) Origin of axial prestretch and residual stress in arteries. Biomech Model Mechanobiol 8:431–46
Chiquet M, Gelman L, Lutz R, Maier S (2009) From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta 20(5):1793–1911
Chiu JJ, Chien S (2011) Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91(1):327–87
Chiu JJ, Usami S, Chien S (2009) Vascular endothelial responses to altered shear stress: pathologic implications for atherosclerosis. Ann Med 41(1):19–28
Choudhury N, Bouchot O, Rouleau L, Tremblay D, Cartier R, Butany J, Mongrain R, Leask RL (2009) Local mechanical and structural properties of healthy and diseased human ascending aorta tissue. Cardiovasc Pathol 18(2):83–91
Chuong CJ, Fung YC (1986) On residual stresses in arteries. J Biomech 108:189–192
Davis EC (1995) Elastic lamina growth in the developing mouse aorta. J Histochem Cytochem 43:1115–23
Davis FM, Luo Y, Avril S, Duprey A, Lu J (2016) Local mechanical properties of human ascending thoracic aneurysms. J Mech Behav Biomed Mater 61:235–49
de Putter S, Wolters BJBM, Ruttena MCM, Breeuwer M, Gerritsen FA, van de Vosse FN (2006) Patient-specific initial wall stress in abdominal aortic aneurysms with a backward incremental method. J Biomech 40:1081–90
Demiray H (1991) A layered cylindrical shell model for an aorta. Int J Eng Sci 29(1):47–54
Dorrington KL, McCrum NG (1977) Elastin as rubber. Biopolymers 16:1201–22
Farzaneh S, Paseta O, Gómez-Benito MJ (2015) Multi-scale finite element model of growth plate damage during the development of slipped capital femoral epiphysis. Biomech Model Mechanobiol 14(2):371–85
Ferruzzi J, Collins MJ, Yeh AT, Humphrey JD (2011) Mechanical assessment of elastin integrity in fibrillin-1-deficient carotid arteries: implications for marfan syndrome. Cardiovasc Res 92(2):287–295
Fung YC (1983) In: Fung YC, Fukada E, Wang JJ (eds) What principle governs the stress distribution in living organism? Biomechanics in China, Japan and USA. Science Press: Beijing
García-Herrera CM, Celentano DJ (2013) Modelling and numerical simulation of the human aortic arch under in vivo conditions. Biomech Model Mechanobiol 12(6):1143–54
Gasser TC, Auer M, Labruto F, Swedenborg J, Roy J (2010) Biomechanical rupture risk assessment of abdominal aortic aneurysms: model complexity versus predictability of finite element simulations. Eur J Vasc Endovasc 40:176–185
Greenwald SE, Moore JE, Rachev A, Kane TPC, Meister JJ (1997) Experimental investigation of the distribution of residual strains in the artery wall. J Biomech Eng 119:438–444
Guccione JM, McCulloch AD, Waldman LK (1991) Passive material properties of intact ventricular myocardium determined from a cylindrical model. J Biomech Eng 113:42–55
Hibbit K, Sorensen (2011) Abaqus: theory manual , 6.11-3 edition
Holzapfel GA (2000) Nonlinear solid mechanics: a continuum approach for engineering. Wiley, Chichester
Holzapfel GA, Gasser TC, Ogden RW (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast 61:1–48
Holzapfel GA, Ogden RW (2010) Constitutive modelling of arteries. Proc R Soc A 466:1551–1597
Horný L, Netuśil M, Vońavková T (2014) Axial prestretch and circumferential distensibility in biomechanics of abdominal aorta. Biomech Model Mechanobiol 13(4):783–799
Humphrey JD (1995) Mechanics of arterial wall: review and directions. Crit Rev Biomed Eng 23(1–2):1–162
Humphrey JD (2002) Cardiovascular solid mechanics: cells, tissues, and organs. Springer, NY
Humphrey JD (2008) Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem Biophys 50(2):53–78
Humphrey JD, Rajagopal KR (2002) A constrained mixture model for growth and remodeling of soft tissues. Math Models Methods Appl Sci 12:407–430
Joldes GR, Miller K, Wittek A, Doyle B (2016) A simple, effective and clinically applicable method to compute abdominal aortic aneurysm wall stress. J Mech Behav Biomed Mater 58:139–48
Karsaj I, Soric J, Humphrey JD (2010) A 3-D framework for arterial growth and remodeling in response to altered hemodynamics. Int J Eng Sci 48(11):1357–72
Langille BL (1996) Arterial remodeling: relation to hemodynamics. Can J Physiol Pharmacol 74:834–41
Lu J, Zhou X, Raghavan ML (2007) Inverse elastostatic stress analysis in pre-deformed biological structures: demonstration using abdominal aortic aneurysms. J Biomech 40(3):693–696
Maas SA, Erdemir A, Halloran JP, Weiss JA (2016) A general framework for application of prestrain to computational models of biological materials. J Mech Behav Biomed Mater 61:499–510
Martufi G, Satriano A, Moore RD, Vorp DA, Di Martino ES (2015) Local quantification of wall thickness and intraluminal thrombus offer insight into the mechanical properties of the aneurysmal aorta. Ann Biomed Eng 43(8):1759–1771
Mousavi SJ, Doweidar MH (2016) Numerical modeling of cell differentiation and proliferation in force-induced substrates via encapsulated magnetic nanoparticles. Comput Methods Programs Biomed 130:106–17
Nevo E, Lanir Y (1989) Structural finite deformation model of the left ventricle during diastole and systole. J Biomech Eng 111(4):342–9
Omens JH, Fung YC (1990) Residual strain in rat left ventricle. Circ Res 66:37–45
Peña E, Martinez MA, Calvo B, Doblaré M (2006) On the numerical treatment of initial strains in biological soft tissues. Int J Numer Methods Eng 68:836–860
Rachev A (1997) Theoretical study of the effect of stress-dependent remodeling on arterial geometry under hypertensive conditions. J Biomech 30(8):819–27
Raghavan ML, Baoshun MA, Filinger MF (2006) Non-invasive determination of zero-pressure geometry of arterial aneurysms. Ann Biomed Eng 34(9):1414–19
Rausch MK, Kuhl E (2013) On the effect of prestrain and residual stress in thin biological membranes. J Mech Phys 61:1955–1969
Riveros F, Chandra S, Finol EA, Gasser TC, Rodriguez JF (2013) A pull-back algorithm to determine the unloaded vascular geometry in anisotropic hyperelastic aaa passive mechanics. Ann Biomed Eng 41(4):694–708
Rodriguez JF, Ruiz C, Doblaré M, Holzapfel G (2008) Mechanical stresses in abdominal aortic aneurysms: influence of diameter, asymmetry, and material anisotropy. ASME J Biomech 130(2):021023
Smoljkić M, Vander Sloten J, Segers P, Famaey N, Famaey N (2015) Non-invasive, energy-based assessment of patient-specific material properties of arterial tissue. Biomech Model Mechanobiol 14(5):1045–1056
Speelman L, Bosboom EM, Schurink GW, Buth J, Breeuwer M, Jacobs MJ, van de Vosse FN (2009) Initial stress and nonlinear material behavior in patient-specific aaa wall stress analysis. J Biomech 42(11):1713–19
Stalhand J (2009) Determination of human arterial wall parameters from clinical data. Biomech Model Mechanobiol 8(2):141–8
Taber LA, Humphrey JD (2001) Stress-modulated growth, residual stress, and vascular heterogeneity. J Biomech Eng 123(6):528–35
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat Rev 3:349–363
Trabelsi O, Davis FM, Rodriguez-Matas JF, Duprey A, Avril S (2015) Patient specific stress and rupture analysis of ascending thoracic aneurysms. J Biomech 48(10):1836–43
Trabelsi O, Duprey A, Favre JP, Avril S (2016) Predictive models with patient specific material properties for the biomechanical behavior of ascending thoracic aneurysms. Ann Biomed Eng 44(1):84–98
Valentín A, Humphrey JD, Holzapfel GA (2013) A finite element-based constrained mixture implementation for arterial growth, remodeling, and adaptation: theory and numerical verification. Int J Numer Methods Biomed Eng 29(8):822–849
von Maltzahn WW, Besdo D, Wiemer W (1981) Elastic properties of arteries: a nonlinear two-layer cylindrical model. J Biomech 14(6):389–97
von Maltzahn WW, Warriyar RG, Keitzer WF (1984) Experimental measurements of elastic properties of media and adventitia of bovine carotid arteries. J Biomech 17(11):839–47
Wang HM, Luo XY, Gao H, Ogden RW, Griffith BE, Berry C, Wang TJ (2014) A modified holzapfel-ogden law for a residually stressed finite strain model of the human left ventricle in diastole. Biomech Model Mechanobiol 13:99–113
Weisbecker H, Unterberger MJ, Holzapfel GA (2015) Constitutive modelling of arteries considering fibre recruitment and three-dimensional fibre distribution. J R Soc Interface. doi:10.1098/rsif.2015.0111
Wilson JS, Humphrey JD (2014) Evolving anisotropy and degree of elastolytic insult in abdominal aortic aneurysms: potential clinical relevance? J Biomech 47(12):2995–3002
Woo SL, Weiss JA, Gomez MA, Hawkins DA (1990) Measurement of changes in ligament tension with knee motion and skeletal maturation. J Biomech Eng 112:46–51
Acknowledgements
The authors are grateful to the European Research Council for grant ERC-2014-CoG BIOLOCHANICS. The authors would also like to thank Nele Famaey, Chiara Bellini and Jay Humphrey for inspiring discussions related to this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mousavi, S.J., Avril, S. Patient-specific stress analyses in the ascending thoracic aorta using a finite-element implementation of the constrained mixture theory. Biomech Model Mechanobiol 16, 1765–1777 (2017). https://doi.org/10.1007/s10237-017-0918-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-017-0918-2