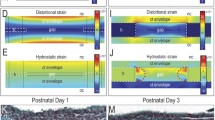
Abstract
How cells utilize instructions provided by genes and integrate mechanical forces generated by tissue growth to produce morphology is a fundamental question of biology. Dermal bones of the vertebrate cranial vault are formed through the direct differentiation of mesenchymal cells on the neural surface into osteoblasts through intramembranous ossification. Here we join a self-organizing Turing mechanism, computational biomechanics, and experimental data to produce a 3D representative model of the growing cerebral surface, cranial vault bones, and sutures. We show how changes in single parameters regulating signaling during osteoblast differentiation and bone formation may explain cranial vault shape variation in craniofacial disorders. A key result is that toggling a parameter in our model results in closure of a cranial vault suture, an event that occurred during evolution of the cranial vault and that occurs in craniofacial disorders. Our approach provides an initial and important step toward integrating biomechanics into the genotype phenotype map to explain the production of variation in head morphology by developmental mechanisms.






Similar content being viewed by others
References
Al-Rekabi Z, Cunningham ML, Sniadecki NJ (2016) Cell mechanics of craniosynostosis. ACS Biomater Sci Eng 3(11):2733–2743. https://doi.org/10.1021/acsbiomaterials.6b00557
Aldridge K, Hill CA, Austin JR, Percival C, Martinez-Abadias N, Neuberger T, Wang Y, Jabs EW, Richtsmeier JT (2010) Brain phenotypes in two FGFR2 mouse models for Apert syndrome. Dev Dyn 239(3):987–997. https://doi.org/10.1002/dvdy.22218
Aspenberg P, Jeppsson C, Economides AN (2001) The bone morphogenetic proteins antagonist noggin inhibits membranous ossification. J Bone Min Res 16(3):497–500. https://doi.org/10.1359/jbmr.2001.16.3.497
Bailón-Plaza A, van der Meulen MCH (2003) Beneficial effects of moderate, early loading and adverse effects of delayed or excessive loading on bone healing. J Biomech 36(8):1069–1077. https://doi.org/10.1016/S0021-9290(03)00117-9
Carroll S, Grenier J, Weatherbee S (2004) From DNA to diversity: molecular genetics and the evolution of animal design. Blackwell, Oxford. https://doi.org/10.1093/jhered/esi101
Carter DR, Blenman PR, Beaupré GS (1988) Correlations between mechanical stress history and tissue differentiation in initial fracture healing. J Orthop Res 6(5):736–748. https://doi.org/10.1002/jor.1100060517
Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC (2003) Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am 85(8):1544–1552
Clendenning DE, Mortlock DP (2012) The BMP ligand Gdf6 prevents differentiation of coronal suture mesenchyme in early cranial development. PLoS ONE 7(5):e36789. https://doi.org/10.1371/journal.pone.0036789
Da Garzón-Alvarado, González A, Gutiérrez ML (2013) Growth of the flat bones of the membranous neurocranium: a computational model. Comput Methods Programs Biomed 112(3):655–64. https://doi.org/10.1016/j.cmpb.2013.07.027
Flaherty K, Singh N, Richtsmeier JT (2016) Understanding craniosynostosis as a growth disorder. Wiley Interdiscip Rev Dev Biol 5(4):429–459. https://doi.org/10.1002/wdev.227
Geris L, Sloten JV, Oosterwyck HV (2010) Connecting biology and mechanics in fracture healing: an integrated mathematical modeling framework for the study of nonunions. Biomech Model Mechanobiol 9(6):713–724. https://doi.org/10.1007/s10237-010-0208-8
Gierer A, Meinhardt H (1972) A theory of biological pattern formation. Kybernetik 12(1):30–39
Hall BK, Miyake T (2000) All for one and one for all: condensations and the initiation of skeletal development. Bioessays 22(2):138–147. https://doi.org/10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4
Ikegame M, Ishibashi O, Yoshizawa T, Shimomura J, Komori T, Ozawa H, Kawashima H (2001) Tensile stress induces bone morphogenetic protein 4 in preosteoblastic and fibroblastic cells, which later differentiate into osteoblasts leading to osteogenesis in the mouse calvariae in organ culture. J Bone Min Res 16(1):24–32. https://doi.org/10.1359/jbmr.2001.16.1.24
Iseki S, Wilkie AOM, Heath JK, Ishimaru T, Eto K, Morriss-Kay GM (1997) Fgfr2 and osteopontin domains in the developing skull vault are mutually exclusive and can be altered by locally applied FGF2. Development 124(17):3375–3384
Kawasaki K, Suzuki T, Weiss KM (2004) Genetic basis for the evolution of vertebrate mineralized tissue. Proc Natl Acad Sci USA 101(31):11356–11361. https://doi.org/10.1073/pnas.0404279101
Kondo S, Shirota H (2009) Theoretical analysis of mechanisms that generate the pigmentation pattern of animals. Semin Cell Dev Biol 20(1):82–89. https://doi.org/10.1016/j.semcdb.2008.10.008
Lai TH, Fong YC, Fu WM, Yang RS, Tang CH (2008) Osteoblasts-derived BMP-2 enhances the motility of prostate cancer cells via activation of integrins. Prostate 68(12):1341–1353. https://doi.org/10.1002/pros.20799
Lee C, Richtsmeier JT, Kraft RH (2017) A computational analysis of bone formation in the cranial vault using a coupled reaction–diffusion–strain model. J Mech Med Biol 17(04):1750073. https://doi.org/10.1142/S0219519417500737
Long F (2011) Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol 13(1):27–38. https://doi.org/10.1038/nrm3254
Marcon L, Diego X, Sharpe J, Müller P (2016) High-throughput mathematical analysis identifies Turing networks for patterning with equally diffusing signals. eLife 5:e14022. https://doi.org/10.7554/eLife.14022
Maul TM, Chew DW, Nieponice A, Vorp DA (2011) Mechanical stimuli differentially control stem cell behavior: morphology, proliferation, and differentiation. Biomech Model Mechanobiol 10(6):939–953. https://doi.org/10.1007/s10237-010-0285-8
Motch Perrine SM, Stecko T, Neuberger T, Jabs EW, Ryan TM, Richtsmeier JT (2017) Integration of brain and skull in prenatal mouse models of Apert and Crouzon syndromes. Front Hum Neurosci 11:369. https://doi.org/10.3389/fnhum.2017.00369
Murray JD (2002) Mathematical biology: I. An introduction. Springer, New York. https://doi.org/10.1007/b98868
O’Leary DD, Chou SJ, Sahara S (2007) Area patterning of the mammalian cortex. Neuron 56(2):252–269. https://doi.org/10.1016/J.NEURON.2007.10.010
Opperman LA (2000) Cranial sutures as intramembranous bone growth sites. Dev Dyn 219(4):472–485. https://doi.org/10.1002/1097-0177(2000)9999:9999<::AID-DVDY1073>3.0.CO;2-F
Palomares KTS, Gleason RE, Mason ZD, Cullinane DM, Einhorn TA, Gerstenfeld LC, Morgan EF (2009) Mechanical stimulation alters tissue differentiation and molecular expression during bone healing. J Orthop Res 27(9):1123–1132. https://doi.org/10.1002/jor.20863
Perlman RL (2016) Mouse models of human disease an evolutionary perspective. Evol Med Public Health 2016(1):170–176
Pillarisetti A, Desai JP, Ladjal H, Schiffmacher A, Ferreira A, Keefer CL (2011) Mechanical phenotyping of mouse embryonic stem cells: increase in stiffness with differentiation. Cell Reprogram 13(4):371–380. https://doi.org/10.1089/cell.2011.0028
Raspopovic J, Marcon L, Russo L, Sharpe J (2014) Digit patterning is controlled by a Bmp–Sox9–Wnt Turing network modulated by morphogen gradients. Science 345(6196):566–570. https://doi.org/10.1126/science.1252960
Rauch F, Lauzier D, Croteau S, Travers R, Glorieux FH, Hamdy R (2000) Temporal and spatial expression of bone morphogenetic protein-2, -4, and -7 during distraction osteogenesis in rabbits. Bone 27(3):453–459. https://doi.org/10.1016/S8756-3282(00)00337-9
Rice DPC, Åberg T, Chan YS, Tang Z, Kettunen PJ, Pakarinen L, Maxson RE, Thesleff I (2000) Integration of FGF and TWIST in calvarial bone and suture development. Development 127(9):1845–1855
Richtsmeier JT, Flaherty K (2013) Hand in glove: brain and skull in development and dysmorphogenesis. Acta Neuropathol 125(4):469–89. https://doi.org/10.1007/s00401-013-1104-y
Sato M, Ochi T, Nakase T, Hirota S, Kitamura Y, Nomura S, Yasui N (1999) Mechanical tension–stress induces expression of bone morphogenetic protein (BMP)-2 and BMP-4, but not BMP-6, BMP-7, and GDF-5 mRNA, during distraction osteogenesis. J Bone Min Res 14(7):1084–1095. https://doi.org/10.1359/jbmr.1999.14.7.1084
Sewda A, White S, Erazo M, Hao K, García-Fructuoso G, Fernández-Rodriguez I, Heuzé Y, Richtsmeier J, Romitti P, Reva B, Jabs E, Peter I (2019) Nonsyndromic craniosynostosis: novel coding variants. Pediatr Res. https://doi.org/10.1038/s41390-019-0274-2
Sidor CA (2001) Simplification as a trend in synapsid cranial evolution. Evolution 55(7):1419–1442. https://doi.org/10.1111/j.0014-3820.2001.tb00663.x
Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A, Lu HH (2013) The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials 34(8):1942–1953. https://doi.org/10.1016/j.biomaterials.2012.11.012
Sugimura K, Shimono K, Uemura T, Mochizuki A (2007) Self-organizing mechanism for development of space-filling neuronal dendrites. PLoS Comput Biol 3(11):e212. https://doi.org/10.1371/journal.pcbi.0030212
Sumanasinghe RD, Bernacki SH, Loboa EG (2006) Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Eng 12(12):3459–3465. https://doi.org/10.1371/journal.pone.0120374
Takagi H, Kaneko K (2002) Pattern dynamics of a multi-component reaction–diffusion system: differntiation of replicating spots. Int J Bifurc Chaos 12(11):2579–2598. https://doi.org/10.1142/S0218127402006084
Ting MC, Wu NL, Roybal PG, Sun J, Liu L, Yen Y, Maxson RE (2009) EphA4 as an effector of Twist1 in the guidance of osteogenic precursor cells during calvarial bone growth and in craniosynostosis. Development (Cambridge) 136(5):855–864. https://doi.org/10.1242/dev.028605
Turing AM (1952) The chemical basis of morphogenesis. Philos Trans R Soc Lond Ser B Biol Sci 237(641):37–72. https://doi.org/10.1098/rstb.1952.0012
Wan M, Cao X (2005) BMP signaling in skeletal development. Biochem Biophys Res Commun 328(3):651–7. https://doi.org/10.1016/j.bbrc.2004.11.067
Wan DC, Pomerantz JH, Brunet LJ, Kim JB, Chou YF, Wu BM, Harland R, Blau HM, Longaker MT (2007) Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J Biol Chem 282(36):26450–26459. https://doi.org/10.1074/jbc.M703282200
Weickenmeier J, Fischer C, Carter D, Kuhl E, Goriely A (2017) Dimensional, geometrical, and physical constraints in skull growth. Phys Rev Lett 118(24):248101. https://doi.org/10.1103/PhysRevLett.118.248101
Westendorf JJ, Kahler RA, Schroeder TM (2004) Wnt signaling in osteoblasts and bone diseases. Gene 341:19–39. https://doi.org/10.1016/j.gene.2004.06.044
Yang L, Dolnik M, Zhabotinsky AM, Epstein IR (2002) Spatial resonances and superposition patterns in a reaction–diffusion model with interacting Turing modes. Phys Rev Lett 88(20):208303. https://doi.org/10.1103/PhysRevLett.88.208303
Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, Iseki S (2008) Cell lineage in mammalian craniofacial mesenchyme. Mech Dev 125(9–10):797–808. https://doi.org/10.1016/j.mod.2008.06.007
Yu HMI, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, Birchmeier W, Hsu W (2005) The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development 132(8):1995–2005. https://doi.org/10.1242/dev.01786
Acknowledgements
Computations for this research were performed on the Pennsylvania State University’s Institute for CyberScience Advanced CyberInfrastructure (ICS-ACI). We acknowledge Matthew Dolack for checking data on github. This work was supported in part through instrumentation funded by a National Science Foundation Grant OCI0821527, a Burroughs-Wellcome Fund 2013 Collaborative Research Travel Grant, Pennsylvania Department of Health using Tobacco Cure Funds, and by the National Institutes of Health Grants R01DE022988 and P01HD078233. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Source code of the reaction–diffusion–strain model and an example case are available at https://github.com/PSUCompBio/skull-growth-modeling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (mp4 2986 KB)
Appendices
Appendix A: Experimental data
High-resolution micro-computed tomography (\(\mu \hbox {CT}\)) and magnetic resonance microscopy (MRM) images of embryonic mice serve as experimental data in our analyses. All use of mice was in compliance with animal welfare guidelines approved by the Pennsylvania State University Institutional Animal Care and Use Committees. \(\mu \hbox {CT}\) images with pixel size and slice thickness ranging from 0.0148 to 0.0168 mm were acquired by the Center for Quantitative Imaging at the Pennsylvania State University (http://eesl.iee.psu.edu/content/cqi) using the HD-600 OMNI-X high-resolution X-ray computed tomography system (Varian Medical Systems, Inc., Lincolnshire, IL). Image data were reconstructed on a \(1024 \times 1024\) pixel grid as a 16-bit tiff but were reduced to 8-bit for image analysis. Isosurfaces were reconstructed to represent all cranial bone at indicated ages based on hydroxyapatite phantoms imaged with the specimens using the software package Avizo 8.1.1 (FEI Company, Inc.). The minimum thresholds used to create the isosurfaces ranged from 70 to \(100\ \hbox {mg}/\hbox {cm}^{3}\) partial density hydroxyapatite. MRM images were acquired by the High Field MRI Facility at the Pennsylvania State University (https://www.imaging.psu.edu/facilities/high-field). The fixed specimens were immersed in 2% Magnevist (Bayer Health Care, Wayne, NJ) phosphor-buffered solution (PBS) for 7–10 days depending upon the embryonic age of the specimen to reduce the T1 and T2 relaxation times. All MRM experiments were conducted on a vertical 14.1 Tesla Varian (Varian Inc., Palo Alto, CA) imaging system with direct drive technology. To prevent drying and to minimize magnetic susceptibility artifacts during scanning, specimens were immersed in fluorinert liquid, FC-43 (3M, St. Paul, MN). A standard imaging experiment with an isotropic resolution of \(80\ \upmu \hbox {m}\) comprised a field of view of \(15.4 \times 14 \times 11\ \hbox {mm}^{3}\) and a matrix size of \(192 \times 132\,(75\%\ {\hbox {partial}}\,{\hbox {Fourier:}}\,176)\ \times 137\). With eight averages and a repetition time of 75 ms (echo time 25 ms), the total scan time was 3 h. MATLAB (The MathWorks, Inc., Natick, MA) was used for image post-processing. By zero-filling all directions by a factor of two, the pixel resolution of a standard imaging experiment was \(40\ \upmu \hbox {m}^3\).
Appendix B: Assumption test
To test our assumptions and examine the effects of each in detail, we compare the simulation results of activator at E17.5 estimated using our computational model with and without each assumption (Fig. 7).
Distribution of osteoblasts at E17.5 from simulation results using various computational models. a Result using a model with only assumptions A1 and A2, without the assumption pertaining to mechanical effects. Bones grow and form sutures through reaction–diffusion process. b Result using a model with assumptions A1, A2, and only the assumption about the mechanical effect on production of activator (A3a). Locations of primary ossification centers are specified by the mechanical effect on the production of activator. c Result using a model with all assumptions A1, A2, and A3. Relative speed of bone growth is achieved by the mechanical effect on cell differentiation (A3b)
Figure 7a shows the results estimated using the model with only A1 and A2; mechanical effects on molecular expression and cell differentiation are not considered. As predicted, primary ossification centers form and bone grows from them with sutures forming between the bones. The effect of the inhibitor appears to play a role in suture formation. The number and locations of bones do not agree with experimental observations and reveal a disorganized pattern of bone formation. Figure 7b shows the results estimated using the model with only A1, A2, and A3a, excluding the assumption of the mechanical effect on cell differentiation. Six bones and associated sutures form in locations similar to our experimental observations as predicted. Consequently, the mechanical effect on the production of activator appears to play a role in specifying the number and location of the primary ossification centers. However, limiting the computation to these assumptions results in a similar growing speed across all bones so that their final volumes are similar, a result that does not match experimental observations. Figure 7c shows the result estimated using the model with all assumptions: A1, A2, A3a, and A3b. The results from the coupled model show that apical growth of the frontal and parietal bones is delayed, as observed in experimental animals. Moreover, growth of the interparietal bone is constrained superoinferiorly relative to the growth of the frontal and parietal bones, modeling what is observed in experimental data. The model reveals that localized restriction of growth of the interparietal is due to reduced accumulated volumetric strain apical to the bone (Fig. 4b). These results reveal the importance of local strain in determining the relative growing speed and final shape of each cranial vault bone.
Rights and permissions
About this article
Cite this article
Lee, C., Richtsmeier, J.T. & Kraft, R.H. A coupled reaction–diffusion–strain model predicts cranial vault formation in development and disease. Biomech Model Mechanobiol 18, 1197–1211 (2019). https://doi.org/10.1007/s10237-019-01139-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-019-01139-z