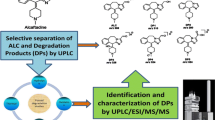
Abstract
The oxidation degradation products in tigecycline were separated and identified by column-switching and online demineralization technique for dual gradient high-performance liquid chromatography (DGLC) combined with Q Orbitrap mass spectrometry for the further improvement of official monographs in pharmacopoeias. Each peak eluted from the non-volatile system was trapped by a switching valve and sent to an LC–MS system using a volatile mobile phase. Thermo Ultimate 3000 DGLC combined with Q Orbitrap MS equipped with electrospray ionization was scanning in positive and negative ion modes at the same time. The one-dimensional analytical column was Welch Ultimate XB-C18 (250 mm × 4.6 mm, 5 μm) with non-volatile salt mobile phase at the flow rate of 1.5 mL min−1 (left pump), and the two-dimensional analytical column was Thermo Acclaim™ 120 C18 (250 mm × 4.6 mm, 5 μm) with a volatile salt mobile phase at the flow rate of 0.3 mL min−1 (right pump). The column temperature was at 30 °C. The detection wavelength was 248 nm. The column-switching and online demineralization technique made it possible to characterize tigecycline in the condition of official standard. The complete fragmentation patterns of impurities were studied and used to obtain information about the structures of these impurities. Mass Frontier software version 7.0 from Thermo Finnigan was used to simulate and study the fragmentation behavior of the described compounds. The structures of six oxidation degradation products in tigecycline were deduced based on the high-resolution MSn data, in which five impurities were novel impurities. The forming mechanisms of oxidation degradation products in tigecycline were also studied. The method solved the problem of incompatibility between non-volatile salt mobile phase and mass spectrometry. It is worthy of widespread use and application, with the advantages of stability and repeatability.








Similar content being viewed by others
References
Chinese state food and drug administration standard (2012) YBH03432012:1–5
Kamel AM, Fouda HG, Brown PR, Munson B (2002) J Am Soc Mass Spectrom 13:543–557
Vartanian VH, Goolsby B, Brodbelt JS (1998) J Am Soc Mass Spectrom 9:1089–1098
Lykkeberg AK, Sengelov G, Cornett C, Tjornelund J, Hansen SH, Halling-Sorensen B (2004) J Pharm Biomed Anal 34:559–567
Cherlet M, De Backer P, Croubels S (2006) J Chromatogr A 1133:135–141
Fioria J, Grassigli G, Filippi P, Gottia R, Cavrini V (2005) J Pharm Biomed Anal 37:979–985
Hu F, Bian K, Liu Y, Su Y, Zhou T, Song X, He L (2014) J Chromatogr A 1368:52–635
Cherlet M, De Backer P, Croubels S (2006) J Chromatogr A 1102:116–124
Gajda A, Posyniak A, Zmudzki J, Tomczyk G (2013) J Chromatogr B 928:113–120
Blanchflower WJ, McCracken RJ, Haggan AS, Kennedy DG (1997) J Chromatogr B 692:351–360
Delepee R, Maume D, Bizec BL, Pouliquen H (2000) J Chromatogr B 748:369–381
Goto T, Ito Y, Yamada S, Matsumoto H, Oka H (2005) J Chromatogr A 1100:193–199
Weiman A, Bojesen G (1999) J Chromatogr B 721:47–54
Cherlet M, Schelkens M, Croubels S, De Backer P (2003) Anal Chim Acta 492:199–213
Chen Y, Schwack W (2013) J Chromatogr A 1312:143–151
Carrasco A, Casado-Terrones S, Swgura-Carretero A, Femandez-Gutierrez A (2008) J Chromatogr A 1195:107–116
Skrásková K, Santos L, Satínsky D, Pena A, Montenegro M, Solich P, Nováková L (2013) J Chromatogr B 927:201–208
Blasco C, Corcia AD, Picó Y (2009) Food Chem 116:1005–1012
Spisso BF, de Araujo MAG, Monteiro MA, Lima AM, Pereira MU, Luiz RA, da Nóbrega AW (2009) Anal Chim Acta 656:72–84
Yuan YZ, Zhang M, Qian W, Yang ZM, Zhao X (2012) Chin Drug Standard 12:106–111
Ji AJ, Saunders JP, Wadgaonkar ND (2007) J Pharm Biomed Anal 44:970–979
Ji AJ, Saunders JP, Amorusi P (2008) J Pharm Biomed Anal 48:866–875
Da Silva LM, Salgado HRN (2012) J Chromatogr Sci 51:192–199
Akin JM, Larkin PJ, Zhu T, Szeliga J (2009) US Patent No. US20090097026:4-16
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Wang, J., Gao, F., Wang, H. et al. Characterization of the Oxidation Degradation Products in Tigecycline by Column-Switching and Online Demineralization Technique for Dual Gradient Liquid Chromatography Combined With Q Orbitrap Mass Spectrometry. Chromatographia 79, 537–545 (2016). https://doi.org/10.1007/s10337-016-3070-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3070-8