Abstract
Purpose
To evaluate the outcomes of a 6-month follow-up after switching to brolucizumab from aflibercept to treat neovascular age-related macular degeneration (AMD) in Japanese patients.
Study design
Retrospective observational study.
Methods
We studied 45 consecutive eyes of 42 patients diagnosed with neovascular AMD, who were switched to intravitreal brolucizumab injection (IVBr) after receiving intravitreal aflibercept injection (IVA) using a treat-and-extend (TAE) regimen. Patients who had brolucizumab-associated intraocular inflammation (IOI) were excluded from the study. The mean changes in the logarithm of the minimum angle of resolution (logMAR) best-corrected visual acuity (BCVA), central foveal thickness (CFT), central choroidal thickness (CCT), and treatment intervals were evaluated at 6 months after the switch to IVBr.
Results
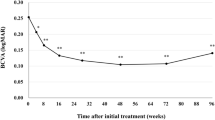
One eye of 1 patient was excluded because of IOI after the switch; 44 eyes of 41 patients were enrolled in this study. The mean logMAR BCVA was maintained throughout the follow-up period when compared with the baseline value (P > .05 at 6 months). However, the mean CFT and CCT at 6 months had decreased significantly (P < .05 and P < .001, respectively). The mean treatment interval was extended from 5.75 to 8.12 weeks.
Conclusion
Switching to brolucizumab from aflibercept using a TAE regimen might be effective for maintaining functional outcomes and extending intervals in Japanese patients with AMD.





Similar content being viewed by others
References
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201.
Hara C, Wakabayashi T, Nishida K. Tachyphylaxis during treatment of exudative age-related macular degeneration with aflibercept. Graefes Arch Clin Exp Ophthalmol. 2019;257:2559–69.
Gasperini JL, Fawzi AA, Khondkaryan A, Lam L, Chong LP, Eliott D, et al. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol. 2012;96:14–20.
Eghoj MS, Sorensen TL. Tachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol. 2012;96:21–3.
Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84.
Bulirsch LM, Sassmannshausen M, Nadal J, Liegl R, Thiele S, Holz FG. Short-term real-world outcomes following intravitreal brolucizumab for neovascular AMD: SHIFT study. Br J Ophthalmol. 2021;10:1136.
Haensli C, Pfister IB, Garweg JG. Switching to brolucizumab in neovascular age-related macular degeneration incompletely responsive to ranibizumab or aflibercept: real-life 6 month outcomes. J Clin Med. 2021;10:2666.
Engelbert M, Zweifel SA, Freund KB. “Treat and extend” dosing of intravitreal antivascular endothelial growth factor therapy for type 3 neovascularization/retinal angiomatous proliferation. Retina. 2009;29:1424–31.
Ho VY, Yeh S, Olsen TW, Bergstrom CS, Yan J, Cribbs BE, et al. Short-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitors. Am J Ophthalmol. 2013;156:23–8.e22.
Mantel I, Gillies MC, Souied EH. Switching between ranibizumab and aflibercept for the treatment of neovascular age-related macular degeneration. Surv Ophthalmol. 2018;63:638–45.
Gale RP, Pearce I, Eter N, Ghanchi F, Holz FG, Schmitz-Valckenberg S, et al. Anatomical and functional outcomes following switching from aflibercept to ranibizumab in neovascular age-related macular degeneration in Europe: SAFARI study. Br J Ophthalmol. 2020;104:493–9.
Ying GS, Kim BJ, Maguire MG, Huang J, Daniel E, et al. Sustained visual acuity loss in the comparison of age-related macular degeneration treatments trials. JAMA Ophthalmol. 2014;132:915–21.
Baumal CR, Spaide RF, Vajzovic L, Freund KB, Walter SD, John V, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127:1345–59.
Mones J, Srivastava SK, Jaffe GJ, Tadayoni R, Albini TA, Kaiser PK, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128:1050–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. Kitajima, None; M. M-Inoue, None; S. Ikeda, None; A. Ito, None; T. Inoue, None; Y. Yanagi, None; K. Kadonosono, None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Maiko Maruyama-Inoue
About this article
Cite this article
Kitajima, Y., Maruyama-Inoue, M., Ikeda, S. et al. Short-term outcomes of switching to brolucizumab in japanese patients with neovascular age-related macular degeneration. Jpn J Ophthalmol 66, 511–517 (2022). https://doi.org/10.1007/s10384-022-00940-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-022-00940-1