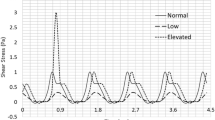
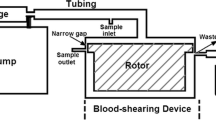
Abstract
Individuals with mechanical heart valve implants are plagued by flow-induced thromboembolic complications, which are undoubtedly caused by platelet activation. Flow fields in or around the affected regions involve brief exposure to pathologically high-shear stresses on the order of 100 to 1000 dyne/cm2. Although high shear is known to activate platelets directly, their subsequent behavior is not known. We hypothesize that the post-high-shear activation behavior of platelets is particularly relevant in understanding the increased thrombotic risk associated with blood-recirculating prosthetic cardiovascular devices. Purified platelets were exposed to brief (5–40 s) periods of high-shear stress, and then exposed to longer periods (15–60 min) of low shear. Their activation state was measured using a prothrombinase-based assay. Platelets briefly exposed to an initial high-shear stress (e.g., 60 dyne/cm2 for 40 s) activate a little, but this study shows that they are now sensitized, and when exposed to subsequent low shear stress, they activate at least 20-fold faster than platelets not initially exposed to high shear. The results show that platelets in vitro exposed beyond a threshold of high-shear stress are primed for subsequent activation under normal cardiovascular circulation conditions, and they do not recover from the initial high-shear insult.







Similar content being viewed by others
References
Akins, C. W. Results with mechanical cardiac valvular prostheses. Ann. Thorac. Surg. 60:1836–1844, 1995.
Anderson, G. H., J. D. Hellums, J. Moake, and C. P. Alfrey, Jr. Platelet response to shear stress: changes in serotonin uptake, serotonin release, and ADP induced aggregation. Thromb. Res. 13:1039–1047, 1978.
Aursnes, I., J. Sundal, and T. Nome. Shear stress activation of platelets with subsequent refractoriness. Thromb. Res. 45:29–37, 1987.
Bahou, W. F., L. Scudder, D. Rubenstein, and J. Jesty. A shear-restricted pathway of platelet procoagulant activity is regulated by IQGAP1. J. Biol. Chem. 279:22571–22577, 2004.
Bluestein, D. Towards optimization of the thrombogenic potential of blood recirculating cardiovascular devices using modeling approaches. Expert. Rev. Med. Devices 3:267–270, 2006.
Bluestein, D., W. Yin, K. Affeld, and J. Jesty. Flow-induced platelet activation in mechanical heart valves. J. Heart Valve Dis. 13:501–508, 2004.
Boreda, R., R. S. Fatemi, and S. E. Rittgers. Potential for platelet stimulation in critically stenosed carotid and coronary arteries. J. Vasc. Invest. 1:26–37, 1995.
Bray, P. F. Platelet hyperreactivity: predictive and intrinsic properties. Hematol. Oncol. Clin. North Am. 21:633–645, v–vi, 2007.
Brown, III, C. H., R. F. Lemuth, J. D. Hellums, L. B. Leverett, and C. P. Alfrey. Response of human platelets to shear stress. Trans. Am. Soc. Artif. Intern. Organs 21:35–39, 1975.
Butchart, E. G., A. Ionescu, N. Payne, J. Giddings, G. L. Grunkemeier, and A. G. Fraser. A new scoring system to determine thromboembolic risk after heart valve replacement. Circulation 108(Suppl 1):II68–II74, 2003.
Butchart, E. G., P. A. Lewis, G. L. Grunkemeier, N. Kulatilake, and I. M. Breckenridge. Low risk of thrombosis and serious embolic events despite low-intensity anticoagulation. Experience with 1,004 Medtronic Hall valves. Circulation 78:I66–I77, 1988.
Colantuoni, G., J. D. Hellums, J. L. Moake, and C. P. Alfrey, Jr. The response of human platelets to shear stress at short exposure times. Trans. Am. Soc. Artif. Intern. Organs 23:626–631, 1977.
Davies, T. A., D. L. Drotts, G. J. Weil, and E. R. Simons. Cytoplasmic Ca2+ is necessary for thrombin-induced platelet activation. J. Biol. Chem. 264:19600–19606, 1989.
Edmunds, Jr., L. H. Thrombotic and bleeding complications of prosthetic heart valves. Ann. Thorac. Surg. 44:430–445, 1987.
Goldsmith, H. L., and V. T. Turitto. Rheological aspects of thrombosis and haemostasis: basic principles and applications. ICTH Report—Subcommittee on Rheology of the International Committee on Thrombosis and Haemostasis. Thromb. Haemost. 55:415–435, 1986.
Grigioni, M., C. Daniele, G. D’Avenio, and V. Barbaro. A discussion on the threshold limit for hemolysis related to Reynolds shear stress. J. Biomech. 32:1107–1112, 1999.
Hathcock, J. J. Flow effects on coagulation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 26:1729–1737, 2006.
Jesty, J., and D. Bluestein. Acetylated prothrombin as a substrate in the measurement of the procoagulant activity of platelets: elimination of the feedback activation of platelets by thrombin. Anal. Biochem. 272:64–70, 1999.
Jesty, J., W. Yin, P. Perrotta, and D. Bluestein. Platelet activation in a circulating flow loop: combined effects of shear stress and exposure time. Platelets 14:143–149, 2003.
Kottke-Marchant, K. Importance of platelets and platelet response in acute coronary syndromes. Cleve. Clin. J. Med. 76(Suppl 1):S2–S7, 2009.
Kroll, M. H., J. D. Hellums, L. V. McIntire, A. I. Schafer, and J. L. Moake. Platelets and shear stress. Blood 88:1525–1541, 1996.
Leytin, V., D. J. Allen, S. Mykhaylov, L. Mis, E. V. Lyubimov, B. Garvey, and J. Freedman. Pathologic high shear stress induces apoptosis events in human platelets. Biochem. Biophys. Res. Commun. 320:303–310, 2004.
Miyazaki, Y., S. Nomura, T. Miyake, H. Kagawa, C. Kitada, H. Taniguchi, Y. Komiyama, Y. Fujimura, Y. Ikeda, and S. Fukuhara. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood 88:3456–3464, 1996.
Nobili, M., J. Sheriff, U. Morbiducci, A. Redaelli, and D. Bluestein. Platelet activation due to hemodynamic shear stresses: damage accumulation model and comparison to in vitro measurements. ASAIO J. 54:64–72, 2008.
Nomura, S. Function and clinical significance of platelet-derived microparticles. Int. J. Hematol. 74:397–404, 2001.
Piccin, A., W. G. Murphy, and O. P. Smith. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 21:157–171, 2007.
Ramstack, J. M., L. Zuckerman, and L. F. Mockros. Shear-induced activation of platelets. J. Biomech. 12:113–125, 1979.
Schulz-Heik, K., J. Ramachandran, D. Bluestein, and J. Jesty. The extent of platelet activation under shear depends on platelet count: differential expression of anionic phospholipid and factor Va. Pathophysiol. Haemost. Thromb. 34:255–262, 2005.
Shankaran, H., P. Alexandridis, and S. Neelamegham. Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood 101:2637–2645, 2003.
Sutera, S. P., and M. H. Mehrjardi. Deformation and fragmentation of human red blood cells in turbulent shear flow. Biophys. J. 15:1–10, 1975.
Travis, B. R., U. M. Marzec, H. L. Leo, T. Momin, C. Sanders, S. R. Hanson, and A. P. Yoganathan. Bileaflet aortic valve prosthesis pivot geometry influences platelet secretion and anionic phospholipid exposure. Ann. Biomed. Eng. 29:657–664, 2001.
Voisin, P., C. Guimont, and J. F. Stoltz. Experimental investigation of the rheological activation of blood platelets. Biorheology 22:425–435, 1985.
Wootton, D. M., and D. N. Ku. Fluid mechanics of vascular systems, diseases, and thrombosis. Annu. Rev. Biomed. Eng. 1:299–329, 1999.
Wurzinger, L. J., R. Opitz, P. Blasberg, and H. Schmid-Schonbein. Platelet and coagulation parameters following millisecond exposure to laminar shear stress. Thromb. Haemost. 54:381–386, 1985.
Yee, D. L., A. L. Bergeron, C. W. Sun, J. F. Dong, and P. F. Bray. Platelet hyperreactivity generalizes to multiple forms of stimulation. J. Thromb. Haemost. 4:2043–2050, 2006.
Yee, D. L., C. W. Sun, A. L. Bergeron, J. F. Dong, and P. F. Bray. Aggregometry detects platelet hyperreactivity in healthy individuals. Blood 106:2723–2729, 2005.
Yin, W., S. Gallocher, L. Pinchuk, R. T. Schoephoerster, J. Jesty, and D. Bluestein. Flow-induced platelet activation in a St. Jude mechanical heart valve, a trileaflet polymeric heart valve, and a St. Jude tissue valve. Artif. Organs 29:826–831, 2005.
Yoganathan, A. P., Z. He, and S. Casey Jones. Fluid mechanics of heart valves. Annu. Rev. Biomed. Eng. 6:331–362, 2004.
Zhang, J. N., A. L. Bergeron, Q. Yu, C. Sun, L. McBride, P. F. Bray, and J. F. Dong. Duration of exposure to high fluid shear stress is critical in shear-induced platelet activation-aggregation. Thromb. Haemost. 90:672–678, 2003.
Zhang, J. N., A. L. Bergeron, Q. Yu, C. Sun, L. V. McIntire, J. A. Lopez, and J. F. Dong. Platelet aggregation and activation under complex patterns of shear stress. Thromb. Haemost. 88:817–821, 2002.
Acknowledgments
The authors would like to thank Philip Chiu, Mohammed Hoque, and Thomas Claiborne for their contributions to the experiments in this study. This study was supported by the American Heart Association (Award no. 0340143N, DB) and the National Institutes of Health (Award no. 1R01EB008004-01, DB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Konstantinos Konstantopoulos oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Sheriff, J., Bluestein, D., Girdhar, G. et al. High-Shear Stress Sensitizes Platelets to Subsequent Low-Shear Conditions. Ann Biomed Eng 38, 1442–1450 (2010). https://doi.org/10.1007/s10439-010-9936-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-9936-2