Abstract
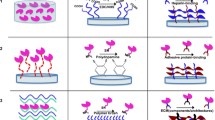
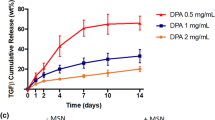
Clinical trials with mesenchymal stem cells (MSCs) have demonstrated potential to treat osteoarthritis, a debilitating disease that affects millions. However, these therapies are often less effective due to heterogeneous MSC differentiation. Kartogenin (KGN), a synthetic small molecule that induces chondrogenesis, has recently been explored to decrease this heterogeneity. KGN has been encapsulated in nanoparticles due to its hydrophobicity. To explore the effect of nanoparticle properties on KGN and MSC interactions, here we fabricated three nanoparticle formulations that vary in hydrophobicity, size, and surface charge using nanoprecipitation: KGN-loaded poly(lactic acid-co-glycolic acid) (PLGA) nanoparticles (hydrophobic surface, negative charge, ~ 167 nm), PLGA–poly(ethylene glycol) (PEG) nanoparticles (hydrophilic surface, positive charge, ~ 297 nm), and PLGA–PEG–hyaluronic acid (HA) nanoparticles (hydrophilic surface, negative charge, ~ 507 nm). We observed differences in KGN loading, release, and suspension stability, with the PLGA particles exhibiting ~ 50% drug loading and PLGA–PEG–HA particles releasing the most KGN. All nanoparticles were found to interact with MSCs with evidence of increased uptake in PLGA–PEG and PLGA–PEG–HA compared with surface association of PLGA particles. Over short times (~ 7 days), MSCs incubated with all KGN-loaded formulations exhibited a similar increase in sulfated glycosaminoglycans, characteristic of chondrogenic differentiation, compared with non-KGN loaded formulations.






Similar content being viewed by others
Abbreviations
- OA:
-
Osteoarthritis
- ECM:
-
Extracellular matrix
- MSCs:
-
Mesenchymal stem cells
- hMSCs:
-
Human mesenchymal stem cells
- KGN:
-
Kartogenin
- CBFβ:
-
Core-binding factor subunit β
- RUNX:
-
Runt-related transcription factor
- TGF-β1:
-
Transforming growth factor beta 1
- EC50 :
-
Half maximal effective concentration
- MW:
-
Molecular weight
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PLGA–PEG:
-
PLGA–poly(ethylene glycol)
- PLGA–PEG–HA:
-
PLGA–PEG–hyaluronic acid
- GAG:
-
Glycosoaminoglycan
- sGAG:
-
Sulfated glycosaminoglycan
- PEG-bis-NH2 :
-
PEG-bis-amine
- PVA:
-
Poly(vinyl alcohol)
- Sulfo-NHS:
-
N-hydroxysulfosuccinimide
- DCC:
-
N,N′-dicyclohexylcarbodiimide
- EDC:
-
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
- DCM:
-
Dichloromethane
- ACN:
-
Acetonitrile
- MES:
-
2-(N-morpholino)ethanesulfonic acid
- FITC:
-
Fluorescein isothiocyanate
- CTAB:
-
Hexadecyltrimethylammonium bromide
- PBS:
-
Phosphate buffered saline
- DMSO:
-
Dimethyl sulfoxide
- EDTA:
-
Ethylenediaminetetraacetic acid disodium salt
- HCl:
-
Hydrochloric acid
- TFA:
-
Trifluoroacetic acid
- CCK-8:
-
Cell Counting Kit-8
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ITS+:
-
Insulin/transferrin/selenium
- HPLC:
-
High performance liquid chromatography
- N2 :
-
Nitrogen
- RT:
-
Room temperature
- 1H-NMR:
-
Proton nuclear magnetic resonance
- DLS:
-
Dynamic light scattering
- PDI:
-
Polydispersity index
- TEM:
-
Transmission electron microscopy
- EE%:
-
Encapsulation efficiency
- DL:
-
Drug loading
- ANOVA:
-
Analysis of variance
References
Barry, F., and M. Murphy. Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 9:584, 2013.
Danhier, F., E. Ansorena, J. M. Silva, R. Coco, A. Le Breton, and V. Préat. PLGA-based nanoparticles: an overview of biomedical applications. J. Control. Release 161:505–522, 2012.
Decker, R. S., E. Koyama, M. Enomoto-Iwamoto, P. Maye, D. Rowe, S. Zhu, P. G. Schultz, and M. Pacifici. Mouse limb skeletal growth and synovial joint development are coordinately enhanced by Kartogenin. Dev. Biol. 395:255–267, 2014.
Fakhari, A., and C. Berkland. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 9:7081–7092, 2013.
Fan, W., J. Li, L. Yuan, J. Chen, Z. Wang, Y. Wang, C. Guo, X. Mo, and Z. Yan. Intra-articular injection of Kartogenin-conjugated polyurethane nanoparticles attenuates the progression of osteoarthritis. Drug Deliv. 25:1004–1012, 2018.
Freitag, J., D. Bates, R. Boyd, K. Shah, A. Barnard, L. Huguenin, and A. Tenen. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy—a review. BMC Musculoskelet. Disord. 17:230, 2016.
Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 7:5577–5591, 2012.
Gumustas, M., C. T. Sengel-Turk, A. Gumustas, S. A. Ozkan, and B. Uslu. Chapter 5: Effect of polymer-based nanoparticles on the assay of antimicrobial drug delivery systems. In: Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics, edited by A. M. Grumezescu. Amsterdam: Elsevier, 2017, pp. 67–108.
Herrera, M. B., B. Bussolati, S. Bruno, L. Morando, G. S. Mauriello-Romanazzi, I. Stamenkovic, L. Biancone, and G. Camussi. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 72:430–441, 2007.
Horkay, F., P. J. Basser, D. J. Londono, A.-M. Hecht, and E. Geissler. Ions in hyaluronic acid solutions. J. Chem. Phys. 131:184902, 2009.
Hsu, H. J., S. Sen, R. M. Pearson, S. Uddin, P. Král, and S. Hong. Poly(ethylene glycol) corona chain length controls end-group-dependent cell interactions of dendron micelles. Macromolecules 47:6911–6918, 2014.
Hu, Q., B. Ding, X. Yan, L. Peng, J. Duan, S. Yang, L. Cheng, and D. Chen. Polyethylene glycol modified PAMAM dendrimer delivery of Kartogenin to induce chondrogenic differentiation of mesenchymal stem cells. Nanomed. Nanotechnol. Biol. Med. 13:2189–2198, 2017.
Jo, C. H., Y. G. Lee, W. H. Shin, H. Kim, J. W. Chai, E. C. Jeong, J. E. Kim, H. Shim, J. S. Shim, I. S. Shin, J. C. Ra, S. Oh, and K. S. Yoon. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells 32:1254–1266, 2014.
Johnson, K., S. Zhu, M. S. Tremblay, J. N. Payette, J. Wang, L. C. Bouchez, S. Meeusen, A. Althage, C. Y. Cho, X. Wu, and P. G. Schultz. A stem cell-based approach to cartilage repair. Science 336:717, 2012.
Kang, M. L., S. Y. Jeong, and G. I. Im. Hyaluronic acid hydrogel functionalized with self-assembled micelles of amphiphilic PEGylated Kartogenin for the treatment of osteoarthritis. Tissue Eng. Part A 23:630–639, 2017.
Kang, M. L., J. E. Kim, and G. I. Im. Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater. 39:65–78, 2016.
Kang, M. L., J. Y. Ko, J. E. Kim, and G. I. Im. Intra-articular delivery of Kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials 35:9984–9994, 2014.
Kang, H., K. Zhang, Q. Pan, S. Lin, D. S. H. Wong, J. Li, W. Y.-W. Lee, B. Yang, F. Han, G. Li, B. Li, and L. Bian. Remote control of intracellular calcium using upconversion nanotransducers regulates stem cell differentiation in vivo. Adv. Funct. Mater. 28:1802642, 2018.
Kong, L., L. Z. Zheng, L. Qin, and K. K. W. Ho. Role of mesenchymal stem cells in osteoarthritis treatment. J. Orthop. Transl. 9:89–103, 2017.
Kristjansson, B., and S. Honsawek. Current perspectives in mesenchymal stem cell therapies for osteoarthritis. Stem Cells Int. 2014. https://doi.org/10.1155/2014/194318.
Li, X., J. Ding, Z. Zhang, M. Yang, J. Yu, J. Wang, F. Chang, and X. Chen. Kartogenin-incorporated thermogel supports stem cells for significant cartilage regeneration. ACS Appl. Mater. Interfaces 8:5148–5159, 2016.
Li, J., W. Y. W. Lee, T. Wu, J. Xu, K. Zhang, D. S. Hong Wong, R. Li, G. Li, and L. Bian. Near-infrared light-triggered release of small molecules for controlled differentiation and long-term tracking of stem cells in vivo using upconversion nanoparticles. Biomaterials 110:1–10, 2016.
Makadia, H. K., and S. J. Siegel. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3:1377–1397, 2011.
Martínez Rivas, C. J., M. Tarhini, W. Badri, K. Miladi, H. Greige-Gerges, Q. A. Nazari, S. A. Galindo Rodríguez, R. Á. Román, H. Fessi, and A. Elaissari. Nanoprecipitation process: from encapsulation to drug delivery. Int. J. Pharm. 532:66–81, 2017.
Oueslati, N., P. Leblanc, C. Harscoat-Schiavo, E. Rondags, S. Meunier, R. Kapel, and I. Marc. CTAB turbidimetric method for assaying hyaluronic acid in complex environments and under cross-linked form. Carbohydr. Polym. 112:102–108, 2014.
Pate, K., and P. Safier. Chemical metrology methods for CMP quality. In: Advances in Chemical Mechanical Planarization (CMP), edited by S. Babu. Cambridge: Woodhead Publishing, 2016, pp. 299–325.
Pittenger, M. F., A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simonetti, S. Craig, and D. R. Marshak. Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147, 1999.
Rezvantalab, S., N. I. Drude, M. K. Moraveji, N. Güvener, E. K. Koons, Y. Shi, T. Lammers, and F. Kiessling. PLGA-based nanoparticles in cancer treatment. Front. Pharmacol. 9:1260, 2018.
Shang, L., K. Nienhaus, and G. U. Nienhaus. Engineered nanoparticles interacting with cells: size matters. J. Nanobiotechnol. 12:5, 2014.
Shi, D., X. Xu, Y. Ye, K. Song, Y. Cheng, J. Di, Q. Hu, J. Li, H. Ju, Q. Jiang, and Z. Gu. Photo-cross-linked scaffold with Kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS Nano 10:1292–1299, 2016.
Soler, R. R., A. Munar, R. F. Soler, X. Peirau, M. Huguet, M. Alberca, A. Sanchez, S. J. Garcia, and L. Orozco. Treatment of knee osteoarthritis with autologous expanded bone marrow mesenchymal stem cells: 50 cases clinical and MRI results at one year follow-up. J. Stem Cell Res. Ther. 2015. https://doi.org/10.4172/2157-7633.1000285.
Vangara, K. K., J. L. Liu, and S. Palakurthi. Hyaluronic acid-decorated PLGA–PEG nanoparticles for targeted delivery of SN-38 to ovarian cancer. Anticancer Res. 33:2425–2434, 2013.
Wakitani, S., T. Goto, S. J. Pineda, R. G. Young, J. M. Mansour, A. I. Caplan, and V. M. Goldberg. Mesenchymal cell-based repar of large, full-thickness defects of articular cartilage. J. Bone Joint Surg. 76A:579–592, 1994.
Wang, E. C., and A. Z. Wang. Nanoparticles and their applications in cell and molecular biology. Integr. Biol. 6:9–26, 2014.
Wittenauer, R., L. Smith, and K. Aden. Background Paper 612 Osteoarthritis. Geneva: World Health Organization, 2013.
Xu, Q., A. Crossley, and J. Czernuszka. Preparation and characterization of negatively charged poly(lactic-co-glycolic acid) microspheres. J. Pharm. Sci. 98:2377–2389, 2009.
Xu, J., J. Li, S. Lin, T. Wu, H. Huang, K. Zhang, Y. Sun, K. W. K. Yeung, G. Li, and L. Bian. Nanocarrier-mediated codelivery of small molecular drugs and siRNA to enhance chondrogenic differentiation and suppress hypertrophy of human mesenchymal stem cells. Adv. Funct. Mater. 26:2463–2472, 2016.
Yadav, A. K., A. Agarwal, G. Rai, P. Mishra, S. Jain, A. K. Mishra, H. Agrawal, and G. P. Agrawal. Development and characterization of hyaluronic acid decorated PLGA nanoparticles for delivery of 5-fluorouracil. Drug Deliv. 17:561–572, 2010.
Yang, W., Y. Zheng, J. Chen, Q. Zhu, L. Feng, Y. Lan, P. Zhu, S. Tang, and R. Guo. Preparation and characterization of the collagen/cellulose nanocrystals/USPIO scaffolds loaded Kartogenin for cartilage regeneration. Mater. Sci. Eng. C 99:1362–1373, 2019.
Zhu, H., N. Mitsuhashi, A. Klein, L. W. Barsky, K. Weinberg, M. L. Barr, A. Demetriou, and G. D. Wu. The role of the hyaloronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells 24:928–935, 2009.
Acknowledgments
The authors acknowledge financial support from Brown University. We thank Dr. Alessia Battigelli from Brown University for her helpful guidance on the chemical syntheses presented. We also thank Kevin Carlson from the Flow Cytometry Core at Brown University and Professor Robert Hurt at Brown for use of his DLS. Finally, we thank Anthony McCormick from Brown University’s Electron Microscopy Core Facility for assistance with TEM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Debra T. Auguste oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Almeida, B., Wang, Y. & Shukla, A. Effects of Nanoparticle Properties on Kartogenin Delivery and Interactions with Mesenchymal Stem Cells. Ann Biomed Eng 48, 2090–2102 (2020). https://doi.org/10.1007/s10439-019-02430-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02430-x