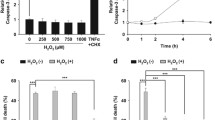
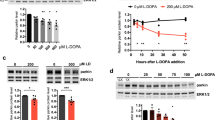
Abstract
Mitochondrial dysfunction caused by oxidative stress and genetic defects have been implicated in the loss of dopaminergic neurons in Parkinson’s disease. However, the key molecular events that provoke neurodegeneration still remain poorly understood. We recently showed that shortly after exposure to oxidative stress, only those cells showing phosphorylation of p53 at Ser-15 subsequently undergo active cell death. To investigate the role of this early p53 signaling response in cell death, 6-hydroxydopamine was used to induce oxidative stress in dopaminergic neurons generated from embryonic stem cells and PC12-D2R cells. Exposure to toxic concentrations of 6-hydroxydopamine induced phosphorylation of p53 at Ser-15 even before cells show mitochondrial permeabilization and apoptosis. We found that 6-hydroxydopamine induced phosphorylation of ataxia telangiectasia mutated (ATM) kinase an event integral to p53 activation and caffeine (ATM kinase inhibitor) inhibited Ser-15 phosphorylation. Phosphorylation of Ser-15 was correlated with enhanced induction and functional activation of p53 manifest as transcription of the pro-apoptotic p53 target Puma. Moreover, inhibition of the p53 abrogated the induction of Puma and promotion of apoptosis due to 6-hydroxydopamine treatments. Thus, these data suggest that activation of p53 signaling immediately after neurotoxin exposure acts as an initiating factor to mediate apoptosis in dopaminergic cells.
Similar content being viewed by others
Abbreviations
- PD:
-
Parkinson’s disease;
- SNc:
-
substantia nigra pars compacta;
- 6-OHDA:
-
6-hydroxydopamine
- ES:
-
embryonic stem cells;
- EB:
-
embryoid body;
- DA:
-
dopamine;
- TH:
-
tyrosine hydroxylase;
- DAT:
-
dopamine transporter;
- Tuj1:
-
β-tubulin type III;
- ERK:
-
extracellular regulated kinase;
- FGF:
-
fibroblast growth factor;
- AA:
-
ascorbic acid;
- MPTP:
-
N-methyl-4-phenyl-1,2,3,6-tetrahydroyridine;
- PFT-α :
-
pifithrin alpha
- ATM:
-
Ataxia Telangiectasia Mutated
References
Nair VD, Yuen T, Olanow CW, Sealfon SC. Early single cell bifurcation of pro- and antiapoptotic states during oxidative stress. J Bio Chem 2004; 279: 27494–27501.
Tanner CM, Ottman R, Goldman SM, et al. Parkinson disease in twins: An etiologic study. JAMA 1999; 281: 341–346.
Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science 2003; 302: 819–822.
Betarbet R, Sherer TB, MacKenzie G, et al. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 2000; 3: 1301–1306.
Burns RS, Chiueh CC, Markey SP, et al. A primate model of parkinsonism: Selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. Proc Natl Acad Sci USA 1983; 80: 4546–4550.
Glinka YY, Youdim MB. Inhibition of mitochondrial complexes I and IV by 6-hydroxydopamine. Eur J Pharmacol 1995; 292: 329–332.
Beal MF. Experimental models of Parkinson’s disease. Nat Rev Neurosci 2001; 2: 325–334.
Dauer W, Przedborski S. Parkinson’s disease: Mechanisms and models. Neuron 2003; 39: 889–909.
Graham DG, Tiffany SM, Bell WR, Jr., Gutknecht WF. Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol Pharmacol 1978; 14: 644–653.
Dodel RC, Du Y, Bales KR, et al. Caspase-3-like proteases and 6-hydroxydopamine induced neuronal cell death. Brain Res Mol Brain Res 1999; 64: 141–148.
Lotharius J, Dugan LL, O’Malley KL. Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J Neurosci 1999; 19: 1284–1293.
Han BS, Hong HS, Choi WS, et al. Caspase-dependent and -independent cell death pathways in primary cultures of mesencephalic dopaminergic neurons after neurotoxin treatment. J Neurosci 2003; 23: 5069–5078.
Ding YM, Jaumotte JD, Signore AP, Zigmond MJ. Effects of 6-hydroxydopamine on primary cultures of substantia nigra: Specific damage to dopamine neurons and the impact of glial cell line-derived neurotrophic factor. J Neurochem 2004; 89: 776–787.
Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000; 408: 307–310.
Morrison RS, Kinoshita Y. The role of p53 in neuronal cell death. Cell Death Differ 2000; 7: 868–879.
Duan W, Zhu X, Ladenheim B, et al. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann Neurol 2002; 52: 597–606.
Mandir AS, Simbulan-Rosenthal CM, Poitras MF, et al. A novel in vivo post-translational modification of p53 by PARP-1 in MPTP-induced parkinsonism. J Neurochem 2002; 83: 186–192.
Blum D, Wu Y, Nissou MF, et al. p53 and Bax activation in 6-hydroxydopamine-induced apoptosis in PC12 cells. Brain Res 1997; 751: 139–142.
Trimmer PA, Smith TS, Jung AB, Bennett JP, Jr. Dopamine neurons from transgenic mice with a knockout of the p53 gene resist MPTP neurotoxicity. Neurodegeneration 1996; 5: 233–239.
Nagy A, Rossant J, Nagy R, et al. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA 1993; 90: 8424–8428.
Lee SH, Lumelsky N, Studer L, et al. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol 2000; 18: 675–679.
Okabe S, Forsberg-Nilsson K, Spiro AC, et al. Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech Dev 1996; 59: 89–102.
Johe KK, Hazel TG, Muller T, et al. Single factors direct the differentiation of stem cells from the fetal and adult nervous system. Genes Dev 1996; 10: 3129–3140.
Nair VD, Olanow W, Sealfon SC. Activation of phosphoinositide 3-kinase by D2 receptor prevents apoptosis in dopaminergic cell lines. Biochem J 2003; 373: 25–32.
Nair VD, Sealfon SC. Agonist specific transactivation of phosphoinositide 3-kinase signaling pathway mediated by the dopamine D2 receptor. J Biol Chem 2003; 278: 47053–47061.
Douhou A, Troadec JD, Ruberg M, et al. Survival promotion of mesencephalic dopaminergic neurons by depolarizing concentrations of K+ requires concurrent inactivation of NMDA or AMPA/kainate receptors. J Neurochem 2001; 78: 163–174.
Kim JH, Auerbach JM, Rodriguez-Gomez JA, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease.Nature 2002; 418: 50–56.
Shim JW, Koh HC, Chang MY, et al. Enhanced in vitro midbrain dopamine neuron differentiation, dopaminergic function, neurite outgrowth, and 1-methyl-4-phenylpyridium resistance in mouse embryonic stem cells overexpressing Bcl-XL. J Neurosci 2004; 24: 843–852.
Hartmann A, Hunot S, Michel PP, et al. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Natl Acad Sci USA 2000; 97: 2875–2880.
Mogi M, Togari A, Kondo T, et al. Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from parkinsonian brain. J Neural Transm 2000; 107: 335–341.
Elkon H, Melamed E, Offen D. Oxidative stress, induced by 6-hydroxydopamine, reduces proteasome activities in PC12 cells: Implications for the pathogenesis of Parkinson’s disease. J Mol Neurosci 2004; 24: 387–400.
Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 1997; 91: 325–334.
Komarov PG, Komarova EA, Kondratov RV, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 1999; 285: 1733–1737.
Culmsee C, Zhu X, Yu QS, et al. A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid beta-peptide. J Neurochem 2001; 77: 220–228.
Villunger A, Michalak EM, Coultas L, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003; 302: 1036–1038.
Mihara M, Erster S, Zaika A, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell 2003; 11: 577–590.
Chipuk JE, Kuwana T, Bouchier-Hayes L, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004; 303: 1010–1014.
Harris SL, Levine AJ. The p53 pathway: Positive and negative feedback loops. Oncogene 2005; 24: 2899–2908.
Canman CE, Lim DS, Cimprich KA, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 1998; 281: 1677–1679.
Chen K, Albano A, Ho A, Keaney JF, Jr. Activation of p53 by oxidative stress involves PDGFbeta receptor-mediated ATM kinase activation. J Biol Chem 2003.
Fornstedt B, Rosengren E, Carlsson A. Occurrence and distribution of 5-S-cysteinyl derivatives of dopamine, dopa and dopac in the brains of eight mammalian species. Neuropharmacology 1986; 25: 451–454.
Blum D, Torch S, Lambeng N, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol 2001; 65: 135–172.
Savitsky K, Bar-Shira A, Gilad S, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 1995; 268: 1749–1753.
Green MH, Marcovitch AJ, Harcourt SA, et al. Hypersensitivity of ataxia-telangiectasia fibroblasts to a nitric oxide donor. Free Radic Biol Med 1997; 22: 343–347.
Chen P, Peng C, Luff J, et al. Oxidative stress is responsible for deficient survival and dendritogenesis in purkinje neurons from ataxia-telangiectasia mutated mutant mice. J Neurosci 2003; 23: 11453–11460.
Canman CE, Lim DS, Cimprich KA, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 1998; 281: 1677–1679.
Morrison RS, Kinoshita Y, Johnson MD, et al. p53-dependent cell death signaling in neurons. Neurochem Res 2003; 28: 15–27.
Owen-Schaub LB, Zhang W, Cusack JC, et al. Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol 1995; 15: 3032–3040.
Muller M, Wilder S, Bannasch D, et al. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med 1998; 188: 2033–2045.
Oda E, Ohki R, Murasawa H, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 2000; 288: 1053–1058.
Yu J, Zhang L, Hwang PM, et al. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 2001; 7: 673–682.
Schuler M, Bossy-Wetzel E, Goldstein JC, et al. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol Chem 2000; 275: 7337–7342.
Tibbetts RS, Brumbaugh KM, Williams JM, et al. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev 1999; 13: 152–157.
Liang SH, Clarke MF. Regulation of p53 localization. Eur J Biochem 2001; 268: 2779–2783.
Zhang Y, Xiong Y. A p53 amino-terminal nuclear export signal inhibited by DNA damage-induced phosphorylation. Science 2001; 292: 1910–1915.
Biswas SC, Ryu E, Park C, et al. Puma and p53 play required roles in death evoked in a cellular model of Parkinson disease. Neurochem Res 2005; 30: 839–845.
Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 2001; 7: 683–694.
Daily D, Barzilai A, Offen D, et al. The involvement of p53 in dopamine-induced apoptosis of cerebellar granule neurons and leukemic cells overexpressing p53. Cell Mol Neurobiol 1999; 19: 261–276.
Tanaka Y, Engelender S, Igarashi S, et al. Inducible expression of mutant alpha-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum Mol Genet 2001; 10: 919–926.
Saha AR, Ninkina NN, Hanger DP, et al. Induction of neuronal death by alpha-synuclein. Eur J Neurosci 2000; 12: 3073–3077.
da Costa CA, Masliah E, Checler F. Beta-synuclein displays an antiapoptotic p53-dependent phenotype and protects neurons from 6-hydroxydopamine-induced caspase 3 activation: Cross-talk with alpha-synuclein and implication for Parkinson’s disease. J Biol Chem 2003; 278: 37330–37335.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nair, V.D. Activation of p53 signaling initiates apoptotic death in a cellular model of Parkinson’s disease. Apoptosis 11, 955–966 (2006). https://doi.org/10.1007/s10495-006-6316-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-6316-3