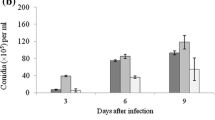
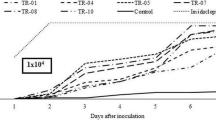
Abstract
The cypress aphid (Cinara cupressi) is listed among the hundred most important invasive pests in the world. In Chile, it was first detected in 2003 and currently is present throughout the country. In the course of a survey of their natural enemies in Chile, three strains of entomopathogenic fungi were isolated. The isolates were identified and tested against the aphid in laboratory experiments. Two further entomopathogenic fungi (ARSEF 5126 and 5128), formulated in the mycoinsecticides Vertalec® and Mycotal®, were used as reference strains. The three Chilean isolates were identified genomically as Lecanicillium attenuatum and were pathogenic to third-instar nymphs. The isolate ARSEF 13279 yielded the lowest overall lethal concentration (LC50), 3 × 105 conidia ml−1 at four days post-inoculation, and the shortest lethal time (LT50), 3.7 days after inoculation with 106 conidia ml−1. The results indicate that the isolates have considerable potential as microbial control agents of the invasive cypress aphid.



Similar content being viewed by others
References
Ansari M, Pope E, Carpenter S, Scholte E, Butt T (2011) Entomopathogenic fungus as a biological control for an important vector of livestock disease: the Culicoides biting midge. PLoS ONE 6(1):e16108
Baldini A, Alvarado A (2008) Manual de Plagas y Enfermedades del Bosque Nativo en Chile. Asistencia para la recuperación y revitalización de los bosques templados de Chile, con énfasis en los Nothofagus caducifolios. FAO/CONAF, Santiago
Baldini A, Oltremari J, Holmgren A (2008) Efecto de Cinara cupressi (Hemiptera: Aphididae) sobre el ciprés de la cordillera (Austrocedrus chilensis) después de aplicar control químico. Cien Inv Agr 35:341–350
Barta M, Cagáň Ľ (2006) Aphid-pathogenic Entomophthorales (their taxonomy, biology and ecology). Biologia 61:543–616
Blackman R, Eastop V (1994) Aphids on the world’s trees: an identification and information guide. CAB International, Wallingford
Buckton G (1881) Monograph of the British aphides, vol 3, pp 46–47
Cabrera-Brandt M, Fuentes-Contreras E, Figueroa CC (2010) Differences in the detoxification metabolism between two clonal lineages of the aphid Myzus persicae (Sulzer) (Hemiptera: Aphididae) reared on tobacco (Nicotiana tabacum l.). Chil J Agric Res 70:567–575
Ciesla W (2011) Forest entomology: a global perspective. Wiley-Blackwell, Oxford
CONAF (2006) Normas de manejo del Sistema Nacional de Áreas Silvestres Protegidas del Estado, Chile
Cortez H (2007) Producción de Lecanicillium (=Verticillium) lecanii en diferentes sustratos y patogenicidad. Agric Téc Méx 33:83–87
Diaz B, Oggerin M, López-Lastra C, Rubio V, Fereres A (2009) Characterization and virulence of Lecanicillium lecanii against different aphid species. BioControl 54:825–835
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
FAO (1991) Exotic Aphid pests of conifers: a crisis in african forestry. In: Proceedings of FAO workshop, 3–6 June 1991 Muguga, Kenya, FAO, Rome, Italy
FAO (2009) Global review of forest pests and diseases: a thematic study prepared in the framework of the global forest resources assessment 2005. FAO forestry paper 156, Rome
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330
Gouy M, Guindon S, Gascuel O (2010) SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Humber RA (1991) Fungal pathogens of aphids. In: Peters D, Webster J, Chlouber C (eds) Aphid-plant interactions: populations to molecules. Agricultural Experiment Station, Division of Agriculture, Oklahoma State University, Stillwater, pp 45–56
Humber RA (2012) Identification of entomopathogenic fungi. In: Lacey L (ed) Manual of techniques in invertebrate pathology. Academic Press (Elsevier Science), Amsterdam, pp 151–187
Humber RA (2016) Seeking stability for research and applied uses of entomopathogenic fungi as biological control agents. J Asia Pac Entomol 19:1019–1025
INFOR (2008) Manejo Integrado: Técnica para la Recuperación del Crecimiento de Austrocedrus chilensis, Santiago
Jandricic SE, Filotas M, Sanderson JP, Wraight SP (2014) Pathogenicity of conidia-based preparations of entomopathogenic fungi against the greenhouse pest aphids Myzus persicae, Aphis gossypii, and Aulacorthum solani (Hemiptera: Aphididae). J Invertebr Pathol 118:34–46
Kim JJ (2007) Influence of Lecanicillium attenuatum on the development and reproduction of the cotton aphid, Aphis gossypii. BioControl 52:789–799
Kim JJ, Roberts DW (2012) The relationship between conidial dose, moulting and insect developmental stage on the susceptibility of cotton aphid, Aphis gossypii, to conidia of Lecanicillium attenuatum, an entomopathogenic fungus. Biocontrol Sci Technol 22:319–331
Kim JJ, Lee MH, Yoon CS, Kim HS, Yoo JK, Kim KC (2001) Control of cotton aphid and greenhouse whitefly with a fungal pathogen. In: Kim YH, Kim JJ, Lee MH, Yoon C-S, Kim H-S, Yoo J-K, Kim K-C (eds) Biological control of greenhouse pests. Food and fertilizer technology center extension bulletin 502, Food and Fertilizer Technology Center, Taipei, pp 7–14
Kim HY, Lee HB, Kim YC, Kim IS (2008) Laboratory and field evaluations of entomopathogenic Lecanicillium attenuatum CNU-23 for control of green peach aphid (Myzus persicae). Microbiol Biotechnol 18:1915–1918
Kope H, Leal I (2006) A new species of Lecanicillium isolated from the white pine weevil, Pissodes strobi. Mycotaxon 94:331–340
Lacey LA, Solter L (2012) Initial handling and diagnosis of diseased invertebrates. In: Lacey L (ed) Manual of techniques in invertebrate pathology. Academic Press (Elsevier Science), Amsterdam, pp 1–14
Lacey LA, Grzywacz D, Shapiro-llan DI, Frutos R, Brownbrigde M, Goettel MS (2015) Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 132:1–41
Leal I, Alfaro RI, Young WL, Kope HH (2008) Molecular characterization of the entomopathogenic fungi Lecanicillium spp. (Deuteromycota: Hyphomycetes) isolated from white pine weevil, Pissodes strobi (Coleoptera: Curculionidae), in British Columbia. Can Entomol 140:168–173
López-Lastra C, Hajek AE, Humber RA (2002) Comparing methods of preservation for cultures of entomopathogenic fungi. Can J Bot 80:1126–1130
Lu L, Cheng B, Du D, Hu X, Peng A, Pu Z, Zhang X, Huang Z, Chen G (2015) Morphological, molecular and virulence characterization of three Lecanicillium species infecting Asian citrus psyllids in Huangyan citrus groves. J Invertebr Pathol 125:45–55
Montalva C, Rojas E, Ruiz C, Lanfranco D (2010a) El pulgón del ciprés en Chile: una revisión de la situación actual y antecedentes del control biológico. Bosque 31:81–88
Montalva C, Gutiérrez M, Rojas E, Lanfranco D, Valenzuela E (2010b) Prospección y determinación de hongos entomopatógenos del pulgón del ciprés, en dos regiones ecológicas de Chile. I. Boletín Micológico 25:1–7
Montalva C, Rojas E, Barta M, Lanfranco D (2013) Biological control agents of cypress aphid present in Chile. In: IV International Symposium on Biological Control of Arthropods, Pucón, Chile
Montalva C, Barta M, Rojas E, Valenzuela E (2013b) Neozygites osornensis sp. nov., a new fungal species causing mortality to the cypress aphid Cinara cupressi in Chile. Mycologia 105:661–669
Montalva C, Arismendi N, Barta M, Rojas E (2014a) Molecular differentiation of recently described Neozygites osornensis (Neozygitales: Neozygitaceae) from two morphologically similar species. J Invertebr Pathol 115:92–94
Montalva C, Barta M, Rojas E, Gutiérrez M, Valenzuela E (2014b) Neozygites species associated with aphids in Chile: current status and new reports. Mycotaxon 129:233–245
Papierok B, Torres B, Arnault M (1984) Contribution to the study of the specificity of the entomopathogenic fungus Zoophthora radicans (Zygomycetes, Entomophthorales). Entomophaga 29:109–119
Rodríguez L, Arredondo H (2007) Teoría y aplicacion del control biologico. Sociedad Mexicana de Control Biológico, Montecillo
Rodriguez RJ, Cullen D, Kurtzman CP, Khachatourians GG, Hegedus DD (2004) Molecular methods for discriminating taxa, monitoring species, and assessing fungal diversity. In: Mueller GM, Bills GF, Foster MS (eds) Biodiversity of fungi: inventory and monitoring methods. Elsevier Academic Press, Burlington, pp 77–102
Saranya S, Ushakumari R, Sosamma J, Babu M (2010) Efficacy of different entomopathogenic fungi against cowpea aphid, Aphis craccivora (Koch). J Biopestic 3:138–142
Shah P, Pell J (2003) Entomopathogenic fungi as biological control agents. Appl Microbiol Biotechnol 61:413–423
Shrestha G, Enkegaard A, Steenberg T (2015) Laboratory and semi-field evaluation of Beauveria bassiana (Ascomycota: Hypocreales) against the lettuce aphid, Nasonovia ribisnigri (Hemiptera: Aphididae). Biol Control 85:37–45
Sorensen JT (2003) Aphids. In: Resh VH, Carde RT (eds) Encyclopedia of insects. Academic Press, San Diego, pp 32–37
Steinkraus DC (2006) Factors affecting transmission of fungal pathogens of aphids. J Invertebr Pathol 92:125–131
Throne JE, Weaker DK, Chew V, Baker JE (1995) Probit analysis of correlated data: multiple observations over time at one concentration. J Econ Entomol 88:1510–1512
van Edhem HF, Harrington R (2007) Aphids as crop pests. CABI, Wallingford
Vu V, Hong S, Kim K (2007) Selection of entomopathogenic fungi for aphid control. J Biosci Bioeng 104:498–505
Watson G, Voegtlin D, Murphy S, Fottit R (1999) Biogeography of the Cinara cupressi complex (Hemiptera: Aphididae) on Cupressaceae, with description of a pest species introduced into Africa. Bull Entomol Res 89:271–283
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Zare R, Gams W (2001) A revision of Verticillium Section Postrata. IV. The genera Lecanicillium and Simplicillium gen. nov. Nova Hedwig. 73:1–50
Zare R, Gams W (2008) A revision of the Verticillium fungicola species complex and its affinity with the genus Lecanicillium. Mycol Res 112:811–824
Acknowledgements
The authors thank the late Dr. Walter Gams who provided important information about the identification of Lecanicillium species; USDA-ARS Collection of Entomopathogenic Fungal Cultures which provided Lecanicillium muscarium ARSEF 5128 and Lecanicillium longisporum ARSEF 5126; PhD supervisor, Dolly Lanfranco, for her continuous support and patience, many steps in this process would not have been accomplished without her help; M.Sc. Mónica Gutierrez for managing the importation and quarantine of foreign strains of Lecanicillium in the Servicio Agrícola y Ganadero (SAG), Laboratorio Regional Osorno; SAG for support of this study in its facilities and DID of the Universidad Austral of Chile Project D-2011-11 for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Nicolai Meyling.
Rights and permissions
About this article
Cite this article
Montalva, C., Valenzuela, E., Barta, M. et al. Lecanicillium attenuatum isolates affecting the invasive cypress aphid (Cinara cupressi) in Chile. BioControl 62, 625–637 (2017). https://doi.org/10.1007/s10526-017-9817-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-017-9817-9