Abstract
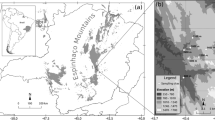
Tropical forests are known for their diverse insect fauna. We aimed to determine the effect and relative importance of latitude, elevation and climatic factors affecting species richness and turnover in euglossine bee assemblages along a gradient of 18° latitude from tropical rainforests to subtropical, deciduous dry forests in Peru and Bolivia. Sixteen forest sites were sampled during the dry season. Variance partitioning techniques were applied to assess the relative effects of the spatial and environmental variables on species richness and composition. Furthermore, we conducted a Species Indicator Analysis to find characteristic species for the biogeographic zones. There was a significant decrease in species richness towards the subtropical area. The best predictors of species richness were precipitation and its consequences on soil properties as well as temperature seasonality. The abundance of euglossines was most closely related to precipitation and soil-pH, but the causal links of abundance to these factors is unclear since soil-pH itself is correlated to a drastic turnover of vegetation structure. Based on the analysis of assemblage composition we propose three different assemblages with a transitional zone at the southern tropical area. The biogeographical distribution of euglossine bees along our study transect appears to be primarily related to climatic conditions and does not reflect the common subdividion of Amazonia into drainage systems.



Similar content being viewed by others
References
Abrahamczyk S, Kessler M (2010) Hummingbird diversity, food niche characters, and assemblage composition along a latitudinal precipitation gradient in the Bolivian lowlands. J Ornithol 151:615–625
Ackerman D (1983a) Specificity and mutual dependency of the orchid-euglossine bee interaction. Biol J Linn Soc 20:301–314
Ackerman D (1983b) Euglossine bee pollination of the orchid Cochleanthes lipscombiae: a food source mimic. Am J Bot 70:830–834
Ackerman D (1983c) Diversity and seasonality of the male euglossine bees (Hymenoptera: Apidae) in central Panamá. Ecol 64:274–283
Ackerman D (1985) Euglossine bees and their nectar hosts. In: D’Arcy WG, Correa MD (eds) The botany and natural history of Panama: La botánica e historia natural de Panamá. Mis Bot Garden, St. Louis
Anjos-Silva EJ, Rebêlo JMM (2006) A new species of Exaerete Hoffmannsegg (Hymenoptera: Apidae: Euglossini) from Brazil. Zootaxa 1105:27–35
Bates JM, Hackett SJ, Cracraft J (1998) Area-relationships in the Neotropical lowlands: an hypothesis based on raw distributions of Passerine birds. J Biogeogr 25:783–793
Bawa KS (1990) Plant pollinator interactions in tropical forests. Ann Rev Ecol Evol Syst 21:399–422
Bembé B (2004) Revision der Euglossa cordata-Gruppe und Untersuchung zur Funktionsmorphologie und Faunistik der Euglossini. Ph.D Thesis, Ludwig- Maximilian-Universität München, Germany
Bonilla-Gómez MA, Nates-Parra G (1992) Abejas euglosinas de Colombia (Hymenoptera: Apidae) I. Claves ilustradas. Caldasia 17:149–172
Braga PIS (1976) Atração de abelhas polinizadoras de Orchidaceae com auxílio de iscas-odores na campina, campinarana e floresta tropical úmida da região de Manaus. Ciência e Cultura 28:767–773
Braga AK (2000) A comunidade de Euglossini de Estação Ecológica de Paulo Faria, Paulo Faria, SP, e comportamento de colecta de fragrãncias pelo machos de Euglossa townsendi Cockerell (Hymenoptera: Apidae: Euglossini). Master thesis, FFCLRP-USP, Ribeirão Preto, Brasil
Brown JH (1984) On the relationship between abundance and distribution of species. Am Nat 124:255–279
Burnham KP, Anderson DR (2004) Multimodel inference—understanding AIC and BIC in model selection. Sociol Meth Res 33:261–304
Búrquez A (1997) Distributional limits of euglossine and meliponine bees (Hymenoptera, Apidae) in northwestern México. Pan-Pac Entomol 73:137
Cameron SA (2004) Phylogeny and biology of the neotropical orchid bees (Euglossini). Ann Rev Entomol 49:377–404
Carranza A, Defeo O, Arim M (2011) Taxonomic relatedness and spatial structure of a shelf benthic gastropod assemblage. Divers Distrib 17:25–34
Colwell RK, Mao CX, Chang J (2004) Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85:2717–2727
Condit R, Pitman NCA, Leigh EG et al (2002) Beta-diversity in tropical forest trees. Science 295:666–669
Dick CW, Roubik DW, Gruber KF et al (2004) Long-distance gene flow and cross-Andean dispersal of lowland rain forest bees (Apidae: Euglossini) revealed by comparative mitochondrial DNA phylogeny. Mol Ecol 13:3775–3785
Dressler RL (1982a) New species of Euglossa II. (Hymenoptera: Apidae). Rev Biol Trop 30:121–129
Dressler RL (1982b) New species of Euglossa III. The Bursigera species group (Hymenoptera: Apidae). Rev Biol Trop 30:131–140
Dressler RL (1982c) New species of Euglossa IV. The cordata and purpurea species groups (Hymenoptera: Apidae). Rev Biol Trop 30:141–150
Dressler RL (1982d) Biology of the orchid bees (Euglossini). Ann Rev Ecol Syst 13:373–394
Dressler RL (1985) Euglossine bees (Hymenoptera: Apidae) of the Tambopata reserved zone, Madre de Dios, Perú. Rev Per Entomol 27:75–79
Ducke A (1902) As espécies Paraenses do gênero Euglossa. Boletim do Museu Paraense 3:561–577
Dufrene M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Duque A, Sánchez M, Cavalier J et al (2002) Different floristic patterns of woody understorey and canopy plants in Colombian Amazonia. J Trop Ecol 18:499–525
Duque A, Phillips JF, von Hildebrand P et al (2009) Distance decay of tree species similarity in protected areas on Terra Firme forests in Colombian Amazonia. Biotropica 41:599–607
Eberhard JR, Bermingham E (2005) Phylogeny and comparative biogeography of Pionopsitta parrots and Pteroglossus toucans. Mol Phylogenet Evol 36:288–304
Fleming TH, Muchala N (2008) Nectar-feeding bird and bat niches in two worlds: pantropical comparisons of vertebrate pollination systems. J Biogeogr 35:764–780
Gentry AH (1988) Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann Mo Bot Gard 75:1–34
Gilbert LE (1980) Food web organization and the conservation of Neotropical diversity. In: Soulé ME, Wilcox BA (eds) Conservation biology: an evolutionary—ecological perspective 1. Sinauer, Sunderland
Hawkins BA, De Vries PJ (2009) Tropical niche conservationism and the species richness gradient of North American butterflies. J Biogeogr 36:1698–1711
Hawkins BA, Field R, Cornell HV et al (2003) Energy, water, and broad-scale geographic pattern of species richness. Ecology 84:3105–3117
Hijmans RJ, Cameron SE, Parra JL et al (2005) Very high resolution interpolated climate surfaces for global land areas. Inter J Climatol 25:1965–1978
Hoorn C, Wesselingh FP, ter Steege H et al (2010) Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330:927–931
Hu L, Li MG, Li Z (2010) Geographical and environmental gradients of lianas and vines in China. Glob Ecol Biogeogr 19:554–561
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press, Princeton
Hubbell SP, Foster RB (1986) Biology, chance, and history and the structure of tropical rain forest tree communities. In: Diamond J, Case TJ (eds) Community ecology. Harper and Row, New York, pp 314–329
Hurtt GC, Pacala SW (1995) The consequences of recruitment limitation: reconciling chance, history and competitive differences between plants. J Theor Biol 176:1–12
Janzen DH (1971) Euglossine bees as long distance pollinators of tropical plants. Science 171:203–205
Justiniano MJ, Fredericksen TS (2000) Phenology of tree species in Bolivian dry forests. Biotropica 32:276–281
Kimsey LS (1982) Systematics of bees of the genus Eufriesea (Hymenoptera, Apidae). Univ Cal Publ Entomol 95
Kimsey LS, Dressler RL (1986) Synonymic species list of Euglossini. Pan-Pacific Entomol 62:229–236
Leyer I, Wesche K (2008) Multivariate Statistik in der Ökologie. Springer, Berlin
McCune B, Mefford MJ (1999) PC-ORD (data analysis software system), version 5. MjM Software Design, Gleneden Beach
Michener CD (1979) Biogeography of the bees. Ann Mo Bot Gard 66:277–342
Michener CD (2007) The bees of the world, 2nd edn. John Hopkins University Press, Baltimore
Minckley RL, Reyes SG (1996) Capture of the orchid bee, Eulaema polychroma (Friese) (Apidae: Euglossini) in Arizona, with notes on the northern distributions of other Mesoamerican bees. J Kans Entomol Soc 69:102–104
Moure JS (1967) A check-list of the known euglossine bees (Hymenoptera, Apidae). Atlas do Simpósio sôbre a Biota Amazônica (Zoologia) 5:395–415
Myers N (1986) Tropical deforestation and a megaextiction spasm. In: Soulé ME (ed) Conservation biology: the science of scarcity and diversity. Sinauer Associates, Sunderland, pp 394–409
Nemésio A, Silveira FA (2006a) Deriving ecological relationships between host and parasitic species—an example with orchid bees. J Biogeogr 33:91–97
Nemésio A, Silveira FA (2006b) Edge effects on the orchid-bee fauna (Hymenoptera: Apidae) at a large remnant of Atlantic forest in southeastern Brazil. Neotropical Entomol 35:313–323
Nemésio A, Silveira FA (2007a) Orchid bee fauna (Hymenoptera: Apidae: Euglossinae) of Atlantic Forest fragments inside an urban area in southeastern Brazil. Neotropical Entomol 36:186–191
Nemésio A, Silveira FA (2007b) Diversity and distribution of orchid bees (Hymenoptera: Apidae: Euglossini) with a revised checklist of species. Neotropical Entomol 36:874–888
Nemésio A, Silveira FA (2009) Orchid bees (Hymenoptera: Apidae) of the Brazilian Atlantic forest. Zootaxa 2041:1–242
Oliveira ML (2006) Três novas espécies de abelhas da Amazônia pertencentes ao gênero Eulaema (Hymenoptera: Apidae: Euglossini). Acta Amaz 36:121–128
Oliveira ML, Campos LAO (1995) Abundância, riqueza e diversidade de abelhas Euglossinae (Hymenoptera: Apidae) em florestas contínuas de terra firme na Amazônia central, Brasil. Rev Brasil Zool 12:547–556
Pitman NCA, Terborgh J, Silman M et al (2001) Dominance and distribution of tree species in upper Amazonian Terra Firme forests. Ecol 82:2101–2117
R Development Core Team (2007) R (data anlysis software system), version 2.11.0. R Foundation for Statistical Computing, Vienna, Austria
Radchenko VG, Pesenko YA (1994) Biology of bees (Hymenoptera, Apoidea). Russian Academy of Sciences, Zoological Institute, St. Petersburg
Ramirez SG, Roubik DW, Skov C, Pierce NE (2010) Phylogeny, diversification patterns and historical biogeography of euglossine orchid bees (Hymenoptera: Apidae). Biol J Lin Soc 100:552–572
Ramírez S, Dressler RL, Ospina AM (2002) Euglossine bees (Hymenoptera: Apidae) from the Neotropical region: a species checklist with notes on their biology. Biota Colombiana 3:7–118
Rangel TFL, Diniz-Filho JAF, Bini LM (2006) Towards an integrated computational tool for spatial analysis in macroecology and biogeography. Glob Ecol Biogeogr 15:321–327
Ren-Cang B, Yu C, Yuan-Man H, Xiu-Zhen L, Hong-Shi H (2008) Sensitivity of coniferous trees to environmental factors at different scales in the Small Xing’ an Mountains, China. Zhiwu Shengtai Xuebao 32:80–87
Roig-Alsina A, Michener CD (1993) Studies of the phylogeny and classification of long-tongued bees (Hymenoptera: Apoidea). Univ Kans Sci Bul 55:123–173
Rosenzweig M (1995) Species diversity in space and time. Cambridge University Press, New York
Roubik DW (1989) Ecology and natural history of tropical bees. Cambridge University Press, Cambridge, UK
Roubik DW (2004a) Long-term studies of solitary bees: what the orchid bees are telling us. In: Freitas BM, Pereira JO (eds) Solitary bees? Conservation, rearing, management for pollination. Imprensa Universitaria, Fortaleza, Brazil, pp 97–103
Roubik DW (2004b) Sibling species of Glossuropoda in the Amazon region (Hymenoptera: Apidae: Euglossini). J Kans Entomol Soc 77:235–253
Roubik DW, Ackerman JD (1987) Long-term ecology of euglossine orchid-bees (Apidae: Euglossini) in Panama. Oecologia 73:321–333
Roubik DW, Hanson PE (2004) Orchid bees of tropical America, 1st edn. INBio, Santo Domingo de Heredia, Costa Rica
Sazima M, Vogel S, Cocucci A, Hausner G (1993) The perfume flowers of Cyphomandra (Solanaceae): pollination by euglossine bees, bellows mechanism, osmophores, and volatiles. Plant Syst Evol 187:55–88
Schwerdtfeger M, Gerlach G, Kaiser R (2002) Anthecology in the neotropical genus Anthurium (Araceae): a preliminary report. Selbyana 23:258–267
Silva JMC, Oren DC (1996) Application of parsimony analysis of endemicity in amazonian biogeography: an example with primates. Biol J Linn Soc 59:427–437
Steyskal GC (1977) History and use of the Mc Phail trap. Fla Entomol 60:11–16
ter Steege H, Pitman N, Sabatier D et al (2003) A spatial model of tree α-diversity and tree density for the Amazon. Biodivers Conerv 12:2255–2277
Toledo M, Poorter L, Peña-Claros M et al (2010) Patterns and determinants of floristic variation across lowland forests of Bolivia. Biotropica 1–9. doi:10.1111/j.1744-7429.2010.00711.x
Tuomisto H, Ruokolainen K, Kalliola R et al (1995) Dissecting Amazonian biodiversity. Science 269:63–66
Tuomisto H, Ruokolainen K, Yli-Halla M (2003a) Dispersal, environment, and floristic variation of western Amazonian forests. Science 299:241–244
Tuomisto H, Ruokulainen K, Aguilar M, Sarmiento A (2003b) Floristic patterns along a 43-km long transect in an Amazonian rain forest. J Ecol 91:743–756
Vasconcelos HL, Vilhena JM, Facure KG et al (2010) Pattern of ant species diversity and turnover across 2000 km of Amazonian floodplain forest. J Biogeogr 37:432–440
Vormisto J, Svenning JC, Hall P et al (2004) Diversity and dominance in palm (Arecaceae) communities in terra firme forests in the western Amazon basin. J Ecol 92:577–588
Wcislo WT, Cane JH (1996) Floral resource utilization by solitary bees (Hymenoptera: Apoidea) and exploitation of their stored foods by natural enemies. Ann Rev Entomol 41:257–286
Werneck FP, Costa GC, Colli GR et al (2010) Revisiting the historical distribution of seasonally dry tropical forests: new insights based on palaeodistribution modelling and palynological evidence. Glob Ecol Biogeogr 20:272–288
Whitfield J (2002) Ecology: neutrality versus the niche. Nature 417:480–481
Whittaker RH, Levin SA (1977) The role of mosaic phenomena in natural communities. Theor Pop Biol 12:117
Wiens JJ, Donoghue MJ (2004) Historical biogeography, ecology and species richness. Trends Ecol Evol 19:639–644
Wiens JJ, Graham CH, Moen DS et al (2006) Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: treefrog trees unearth the roots of high tropical diversity. Am Nat 168:579–596
Williams NH, Dressler RL (1982) Euglossine pollination of Spathyphyllum (Araceae). Selbyana 1:349–356
Williams JW, Jackson ST, Kutzbach JE (2007) Projected distributions of novel and disappearing climates by 2100 AD. Proc Nat Acad Sci 104:5738–5742
Wittmann F, Schöngart J, Montero JC et al (2006) Tree species composition and diversity gradients in white-water forests across the Amazon basin. J Biogeogr 33:1334–1347
Acknowledgements
We thank Y. Gareca, C. Hamel, S. K. Herzog, S. Reichle, V. Sandoval and J. Q. Vidoz for their support and advice during field work. We are grateful to the Botanical Garden in Santa Cruz, the University of Cochabamba, Prometa, the municipal governments of Villa Tunari and Río Seco, and R. Clarke Gemuseus for the permission to work on their land, and to G. Lamas, the National Herbarium at La Paz, the Museum for Natural History, Lima, Peru, as well as the Ministry of the Environment and the INRENA for supporting our study. Furthermore, we thank B. Bembé and G. Gerlach for technical advice, C. Rasmussen and B. Bembé for the support and advice in identifying the specimens, J. Diller for the kind support and advice in the preparation phase, S. Sylvester for reviewing the manuscript, and M. Schwerdtfeger for the baiting agents. Funding was provided by the Konrad-Adenauer-Stiftung and the DFG (Deutsche Forschungsgemeinschaft).
Author information
Authors and Affiliations
Corresponding author
Appendix
Rights and permissions
About this article
Cite this article
Abrahamczyk, S., Gottleuber, P., Matauschek, C. et al. Diversity and community composition of euglossine bee assemblages (Hymenoptera: Apidae) in western Amazonia. Biodivers Conserv 20, 2981–3001 (2011). https://doi.org/10.1007/s10531-011-0105-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-011-0105-1