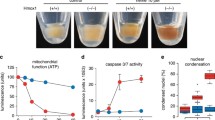
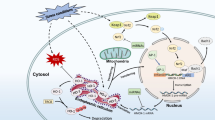
Abstract
Hemopexin (HPX) binds heme tightly, thus protecting cells from heme toxicity during hemolysis, trauma and ischemia-reperfusion injury. Heme uptake via endocytosis of heme-HPX followed by heme catabolism by heme oxygenase-1 (HMOX1) raises regulatory iron pools, thus linking heme metabolism with that of iron. Normal iron homeostasis requires copper-replete cells. When heme-HPX induces HMOX1, the copper-storing metallothioneins (MTs) are also induced whereas the copper-responsive copper chaperone that delivers copper to Cu, Zn superoxide dismutase, CCS1, is decreased; both are known responses when cellular copper levels rise. Endocytosis of heme-HPX is needed to regulate CCS1 since the signaling ligand cobalt-protoporphyrin (CoPP)-HPX, which does not induce HMOX1 but does co-localize with heme-HPX in endosomes, also decreased CCS1. These observations support that heme-HPX mobilizes copper in cells. The regulation of both hmox1 and mt1 is prevented by the copper-chelator, bathocuproinedisulfonate (BCDS), but not uptake of heme-AlexaFluor-labeled HPX into endosomes. Supporting a role for copper in HMOX1 regulation by heme-HPX, nutritional copper deficiency generated by tetraethylene pentamine or 232 tetraamine prevented HMOX1 induction. Using conditions that mimic maturing endosomes, we found that copper prevents rebinding of heme to apo-HPX. A model is presented in which copper endocytosis together with that of heme-HPX provides a means to facilitate heme export from HPX in the maturing endosomes: heme is needed for hmox1 transcription, while cytosolic copper and CCS1 provide a link for the known simultaneous regulation of hmox1 and mt1 by heme-HPX.






Similar content being viewed by others
References
Alam J, Smith A (1989) Receptor-mediated transport of heme by hemopexin regulates gene expression in mammalian cells. J Biol Chem 264:17637–17640
Alam J, Smith A (1992) Heme-hemopexin-mediated induction of metallothionein gene expression. J Biol Chem 267(23):16379–16384
Alam J, Killeen E, Gong P, Naquin R, Hu B, Stewart D, Ingelfinger JR, Nath KA (2003) Heme activates the heme oxygenase-1 gene in renal epithelial cells by stabilizing Nrf2. Am J Physiol Renal Physiol 284(4):F743–F752
Andrews NC (1999) The iron transporter DMT1. Int J Biochem Cell Biol 31(10):991–994. doi:10.1016/S1357-2725(99)00065-5
Arredondo M, Munoz P, Mura CV, Nunez MT (2003) DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am J Physiol Cell Physiol 284(6):C1525–C1530
Bertinato J, L’Abbe MR (2003) Copper modulates the degradation of copper chaperone for Cu, Zn superoxide dismutase by the 26 S proteosome. J Biol Chem 278(37):35071–35078. doi:10.1074/jbc.M302242200
Bittel D, Dalton T, Samson SL, Gedamu L, Andrews GK (1998) The DNA binding activity of metal response element-binding transcription factor-1 is activated in vivo and in vitro by zinc, but not by other transition metals. J Biol Chem 273(12):7127–7133. doi:10.1074/jbc.273.12.7127
Bolton H, Girvin DC, Plymale AE, Harvey SD, Workman DJ (1996) Degradation of metal-nitrilotriacetate complexes by Chelatobacter heintzii. Environ Sci Technol 30:931–938. doi:10.1021/es950397k
Brown SB, Lantzke IR (1969) Solution structures of ferrihaem in some dipolar aprotic solvents and their binary aqueous mixtures. Biochem J 115(2):279–285
Chung J, Prohaska JR, Wessling-Resnick M (2004) Ferroportin-1 is not upregulated in copper-deficient mice. J Nutr 134(3):517–521
Clague MJ (1998) Molecular aspects of the endocytotic pathway. Biochem J 336:271–282
Cobine PA, Pierrel F, Winge DR (2006) Copper trafficking to the mitochondrion and assembly of copper metalloenzymes. Biochim Biophys Acta 1763(7):759–772. doi:10.1016/j.bbamcr.2006.03.002
Conrad CC, Grabowski DT, Walter CA, Sabia M, Richardson A (2000) Using MT (−/−) mice to study metallothionein and oxidative stress. Free Radic Biol Med 28(3):447–462. doi:10.1016/S0891-5849(99)00263-4
Dalton TP, Bittel D, Andrews GK (1997) Reversible activation of mouse metal response element-binding transcription factor 1 DNA binding involves zinc interaction with the zinc finger domain. Mol Cell Biol 17(5):2781–2789
Danzeisen R, Araya M, Harrison B, Keen C, Solioz M, Thiele D, McArdle HJ (2007) How reliable and robust are current biomarkers for copper status? Br J Nutr 98:1–8
Davies DM, Smith A, Muller-Eberhard U, Morgan WT (1979) Hepatic subcellular metabolism of heme from heme-hemopexin: incorporation of iron into ferritin. Biochem Biophys Res Commun 91:1504–1511. doi:10.1016/0006-291X(79)91235-X
Escriba PV, Morales P, Smith A (2002) Membrane phospholipid reorganization differentially regulates metallothionein and heme oxygenase by heme-hemopexin. DNA Cell Biol 21:355–364. doi:10.1089/104454902753759762
Eskew JD, Vanacore RM, Sung L, Morales PJ, Smith A (1999) Cellular protection mechanisms against extracellular heme: heme-hemopexin, but not free heme, activates the N-terminal c-Jun kinase. J Biol Chem 274(2):638–648. doi:10.1074/jbc.274.2.638
Flaherty MM, Rish KR, Smith A, Crumbliss AL (2007) An investigation of hemopexin redox properties by spectroelectrochemistry: biological relevance for heme uptake. Biometals 21(3):259–271
Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112. doi:10.1146/annurev.bi.64.070195.000525
Garrick LM, Dolan KG, Romano MA, Garrick MD (1999) Non-transferrin-bound iron uptake in Belgrade and normal rat erythroid cells. J Cell Physiol 178(3):349–358. doi:10.1002/(SICI)1097-4652(199903)178:3<349::AID-JCP9>3.0.CO;2-R
Gutteridge JM, Smith A (1988) Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. Biochem J 256:861–865
Han O, Wessling-Resnick M (2002) Copper repletion enhances apical iron uptake and transepithelial iron transport by Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 282(3):G527–G533
Heuchel R, Radtke F, Georgiev O, Stark G, Aguet M, Schaffner W (1994) The transcription factor MTF–1 is essential for basal and heavy metal- induced metallothionein gene expression. EMBO J 13(12):2870–2875
Hopkins RG, Failla ML (1999) Transcriptional regulation of interleukin-2 gene expression is impaired by copper deficiency in Jurkat human T lymphocytes. J Nutr 129(3):596–601
Hunt RC, Handy I, Smith A (1996) Heme-mediated reactive oxygen species toxicity to retinal pigment epithelial cells is reduced by hemopexin. J Cell Physiol 168(1):81–86. doi:10.1002/(SICI)1097-4652(199607)168:1<81::AID-JCP10>3.0.CO;2-S
Jiang H, Daniels PJ, Andrews GK (2003) Putative zinc-sensing zinc fingers of metal-response element-binding transcription factor-1 stabilize a metal-dependent chromatin complex on the endogenous metallothionein-I promoter. J Biol Chem 278(32):30394–30402. doi:10.1074/jbc.M303598200
Johnson MB, Enns CA (2004) Diferric transferrin regulates transferrin receptor 2 protein stability. Blood 104(13):4287–4293. doi:10.1182/blood-2004-06-2477
Ledford BE, Davis DF (1983) Kinetics of serum protein secretion by cultured hepatoma cells evidence for multiple secretory pathways. J Biol Chem 258:3304–3308
Lee J, Pena MM, Nose Y, Thiele DJ (2002) Biochemical characterization of the human copper transporter Ctr1. J Biol Chem 277(6):4380–4387. doi:10.1074/jbc.M104728200
Linder MC (1991) Biochemistry of copper. Plenum Press, NewYork
Linder MC (2002) Biochemistry and molecular biology of copper in mammals. Humana Press Inc., Totowa
MacKenzie B, Takanaga H, Hubert N, Rolfs A, Hediger MA (2007) Functional properties of multiple isoforms of human divalent metal-ion transporter 1 (DMT-1). Biochem J 403(1):59–69. doi:10.1042/BJ20061290
Margalit R, Rotenberg M (1984) Thermodynamics of porphyrin dimerization in aqueous solutions. Biochem J 219(2):445–450
Mauk MR, Rosell FI, Lelj-Garolla B, Moore GR, Mauk AG (2005) Metal ion binding to human hemopexin. Biochemistry 44(6):1864–1871. doi:10.1021/bi0481747
Mauk MR, Rosell FI, Mauk AG (2007) Structural modelling of metal ion binding to human haemopexin. Nat Prod Rep 24(3):523–532. doi:10.1039/b604184c
Morgan WT, Muller-Eberhard U (1972) Interactions of porphyrins with rabbit hemopexin. J Biol Chem 247:7181–7187
Morgan WT, Smith A (1984) Domain structure of rabbit hemopexin. Isolation and characterization of a heme-binding glycopeptide. J Biol Chem 259:12001–12006
Morgan W, Smith A (2001) Binding and transport of iron-porphyrins by hemopexin. In: Mauk G, Sykes AG (eds) Advances in inorganic chemistry, vol 51. Academic Press, New York
Narayanan VS, Fitch CA, Levenson CW (2001) Tumor suppressor protein p53 mRNA and subcellular localization are altered by changes in cellular copper in human HepG2 cells. J Nutr 131(5):1427–1432
Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE, Fleming MD (2005) Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet 37(11):1264–1269. doi:10.1038/ng1658
Okazaki H, Taketani S, Kohno H, Tokunaga R, Kobayashi Y (1989) The hemopexin receptor on the cell surface of human polymorphonuclear leukocytes. Cell Struct Funct 14:129–140
Paoli M, Anderson BF, Baker HM, Morgan WT, Smith A, Baker EN (1999) Crystal structure of hemopexin reveals a novel high-affinity heme site formed between two beta-propeller domains. Nat Struct Biol 6(10):926–931. doi:10.1038/13294
Petris MJ, Smith K, Lee J, Thiele DJ (2003) Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem 278(11):9639–9646. doi:10.1074/jbc.M209455200
Picard V, Govoni G, Jabado N, Gros P (2000) Nramp 2 (DCT1/DMT1) expressed at the plasma membrane transports iron and other divalent cations into a calcein-accessible cytoplasmic pool. J Biol Chem 275(46):35738–35745. doi:10.1074/jbc.M005387200
Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV (1999) Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284(5415):805–808. doi:10.1126/science.284.5415.805
Rae TD, Torres AS, Pufahl RA, O’Halloran TV (2001) Mechanism of Cu, Zn-superoxide dismutase activation by the human metallochaperone hCCS. J Biol Chem 276(7):5166–5176. doi:10.1074/jbc.M008005200
Rees EM, Thiele DJ (2007) Identification of a vacuole-associated metalloreductase and its role in CTR2-mediated intracellular copper mobilization. J Biol Chem 282:21629–21638
Ren Y, Smith A (1995) Mechanism of metallothionein gene regulation by heme-hemopexin. Roles of protein kinase C, reactive oxygen species, and cis-acting elements. J Biol Chem 270(41):23988–23995. doi:10.1074/jbc.270.41.23988
Rish KR, Swartzlander R, Sadikot TN, Berridge MV, Smith A (2007) Evidence that heme and heme-hemopexin interact with cell growth-associated plasma membrane electron transport. Biochem Biophys Acta Bioenerg 1767(9):1107–1117. doi:10.1016/j.bbabio.2007.06.003
Rosell FI, Mauk MR, Mauk AG (2005) pH- and metal ion-linked stability of the hemopexin-heme complex. Biochemistry 44(6):1872–1879. doi:10.1021/bi0480077
Sandvig K, Grimmer S, Lauvrak SU, Torgersen ML, Skretting G, van Deurs B, Iversen TG (2002) Pathways followed by ricin and Shiga toxin into cells. Histochem Cell Biol 117(2):131–141. doi:10.1007/s00418-001-0346-2
Schmidt PJ, Ramos-Gomez M, Culotta VC (1999) A gain of superoxide dismutase (SOD) activity obtained with CCS, the copper metallochaperone for SOD1. J Biol Chem 274(52):36952–36956. doi:10.1074/jbc.274.52.36952
Smith A (1999) Assessment of roles of redox-active iron and copper in heme uptake and gene regulation by heme-hemopexin. In: Ferreira GC, Moura JJG, Franco R (eds) Inorganic biochemistry and regulatory mechanisms of iron metabolism, chap 5. Wiley-VCH Publishing Co, Germany
Smith A, Hunt RC (1990) Hemopexin joins transferrin as representative members of a distinct class of receptor-mediated endocytic transport systems. Eur J Cell Biol 53:234–245
Smith A, Ledford BE (1988) Expression of the haemopexin-transport system in cultured mouse hepatoma cells. Links between haemopexin and iron metabolism. Biochem J 256:941–950
Smith A, Morgan WT (1978) Transport of heme by hemopexin to the liver: evidence for receptor-mediated uptake. Biochem Biophys Res Commun 84:151–157. doi:10.1016/0006-291X(78)90276-0
Smith A, Morgan WT (1979) Haem transport to the liver by haemopexin. Receptor-mediated uptake with recycling of the protein. Biochem J 182:47–54
Smith A, Morgan WT (1981) Hemopexin-mediated transport of heme into isolated rat hepatocytes. J Biol Chem 256:10902–10909
Smith A, Alam J, Escriba PV, Morgan WT (1993) Regulation of heme oxygenase and metallothionein gene expression by the heme analogs, cobalt-, and tin-protoporphyrin. J Biol Chem 268(10):7365–7371
Smith A, Eskew JD, Borza CM, Pendrak M, Hunt RC (1997) Role of heme-hemopexin in human T-lymphocyte proliferation. Exp Cell Res 232(2):246–254. doi:10.1006/excr.1997.3526
Sonawane ND, Thiagarajah JR, Verkman AS (2002) Chloride concentration in endosomes measured using a ratioable fluorescent Cl- indicator: evidence for chloride accumulation during acidification. J Biol Chem 277(7):5506–5513. doi:10.1074/jbc.M110818200
Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Igarashi K (2004) Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc Natl Acad Sci USA 101(6):1461–1466. doi:10.1073/pnas.0308083100
Sung L, Morales P, Shibata M, Shipulina N, Smith A (2000a) Defenses against extracellular heme-mediated oxidative damage: use of iron and copper chelators to investigate the role of redox active iron, copper and heme in the hemopexin heme transport system. In: Badman DG, Bergeron RJ, Brittenham GM (eds) Iron chelators: new developmental strategies. Saratoga Publishing Group, Saratoga, Florida, USA, pp 67–86
Sung L, Womack M, Shipulina N, Morales P, Smith A (2000b) Cell surface events for metallothionein-1 and heme oxygenase-1 regulation by the hemopexin heme transport system. Antioxid Redox Signal 2(4):753–765. doi:10.1089/ars.2000.2.4-753
Tapia L, Gonzalez-Aguero M, Cisternas MF, Suazo M, Cambiazo V, Uauy R, Gonzalez M (2004) Metallothionein is crucial for safe intracellular copper storage and cell survival at normal and supra-physiological exposure levels. Biochem J 378(Pt 2):617–624. doi:10.1042/BJ20031174
Tolosano E, Hirsch E, Patrucco E, Camaschella C, Navone R, Silengo L, Altruda F (1999) Defective recovery and severe renal damage after acute hemolysis in hemopexin-deficient mice. Blood 94(11):3906–3914
Tolosano E, Fagoonee S, Hirsch E, Berger FG, Baumann H, Silengo L, Altruda F (2002) Enhanced splenomegaly and severe liver inflammation in haptoglobin/hemopexin double-null mice after acute hemolysis. Blood 100(12):4201–4208. doi:10.1182/blood-2002-04-1270
Tycko B, Maxfield FR (1982) Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell 28(3):643–651. doi:10.1016/0092-8674(82)90219-7
Uauy R, Olivares M, Gonzalez M (1998) Essentiality of copper in humans. Am J Clin Nutr 67(5 Suppl):952S–959S
van Renswoude J, Bridges KR, Harford JB, Klausner RD (1982) Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci USA 79(20):6186–6190. doi:10.1073/pnas.79.20.6186
Vanacore R, Eskew J, Morales P, Sung L, Smith A (2000) Role for copper in transient oxidation and nuclear translocation of MTF-1, but not of NFκB, by the hemopexin heme transport system. Antioxid Redox Signal 2(4):739–752. doi:10.1089/ars.2000.2.4-739
Vretblad P, Hjorth R (1977) The use of wheat-germ lectin-Sepharose for the purification of human haemopexin. Biochem J 167:759–764
Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ (1999) Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet 21(2):195–199. doi:10.1038/5979
Waalkes MP, Harvey MJ, Klaassen CD (1984) Relative in vitro affinity of hepatic metallothionein for metals. Toxicol Lett 20(1):33–39. doi:10.1016/0378-4274(84)90179-6
West EC, Prohaska JR (2004) Cu, Zn-superoxide dismutase is lower and copper chaperone CCS is higher in erythrocytes of copper-deficient rats and mice. Exp Biol Med (Maywood) 229(8):756–764
Acknowledgments
The authors wish to thank Dr. M. Ferrari (University of Missouri-K.C.) for his help with confocal microscopy; and are greatly indebted to Dr. J. Prohaska (University of Minnesota) for affinity purified antibodies to CCS1 and to Dr. R.C. Hider (Kings College, London, UK.) for the physical constants for the metal chelators, for the characterization of Cu-NTA complexes (speciation plots and the calculations of free copper from Cu-NTA complexes at different pHs). Drs. G. Anderson (QMIR, Brisbane, Australia), R.C. Hider and J. Price (University of Missouri-K.C.) are also thanked for reading the manuscript and for their helpful comments. This research was supported by the National Institutes of Health (R21DK 64363 to A.S.) and by a grant from the University of Missouri Research Board (A.S.). The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authorship contributions
R.L. and K.R. carried out experiments, participated in the data statistical analyses and helped set up the figures; R.H. and J.H. carried out the fluorescence microscopy experiments and R.H. made the microscopy figures. A.S. defined the experimental strategy, designed and supervised the research, interpreted data and wrote the manuscript.
Rights and permissions
About this article
Cite this article
Smith, A., Rish, K.R., Lovelace, R. et al. Role for copper in the cellular and regulatory effects of heme-hemopexin. Biometals 22, 421–437 (2009). https://doi.org/10.1007/s10534-008-9178-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-008-9178-z