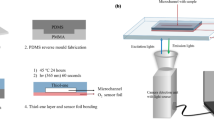
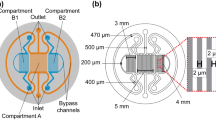
Abstract
Microfluidic bioreactors fabricated from highly gas-permeable poly(dimethylsiloxane) (PDMS) materials have been observed, somewhat unexpectedly, to give rise to heterogeneous long term responses along the length of a perfused mammalian cell culture channel, reminiscent of physiologic tissue zonation that arises at least in part due to oxygen gradients. To develop a more quantitative understanding and enable better control of the physical-chemical mechanisms underlying cell biological events in such PDMS reactors, dissolved oxygen concentrations in the channel system were quantified in real time using fluorescence intensity and lifetime imaging of an oxygen sensitive dye, ruthenium tris(2,2’-dipyridyl) dichloride hexahydrate (RTDP). The data indicate that despite oxygen diffusion through PDMS, uptake of oxygen by cells inside the perfused PDMS microchannels induces an axial oxygen concentration gradient, with lower levels recorded in downstream regions. The oxygen concentration gradient generated by a balance of cellular uptake, convective transport by media flow, and permeation through PDMS in our devices ranged from 0.0003 (mg/l)/mm to 0.7 (mg/l)/mm. The existence of such steep gradients induced by cellular uptake can have important biological consequences. Results are consistent with our mathematical model and give insight into the conditions under which flux of oxygen through PDMS into the microchannels will or will not contribute significantly to oxygen delivery to cells and also provide a design tool to manipulate and control oxygen for cell culture and device engineering. The combination of computerized microfluidics, in situ oxygen sensing, and mathematical models opens new windows for microphysiologic studies utilizing oxygen gradients and low oxygen tensions.




Similar content being viewed by others
References
J.W. Allen and S.N. Bhatia, Formation of steady-state oxygen gradients in vitro: application to liver zonation. Biotechnol. Bioeng. 82, 253–262 (2003).
J.W. Allen, S.R. Khetani, and S.N. Bhatia, In vitro zonation and toxicity in a hepatocyte bioreactor. Toxicol. Sci. 84: 110–119 (2005).
S. Andreescu, O.A. Sadik, D.W. McGee, and S. Suye, Autonomous multielectrode system for monitoring the interactions of isoflavonoids with lung cancer cells. Anal. Chem. 76, 2321–2330 (2004).
R. Benninger, O. Hofmann, J. McGinty, J. Requejo-Isidro, I. Munro, M. Neil, A. deMello, and P. French, Time-resolved fluorescence imaging of solvent interactions in microfluidic devices. Opt. Express. 13, 6275–6285 (2005).
D.A. Chang-Yen and B.K. Gale, An integrated optical oxygen sensor fabricated using rapid-prototyping techniques. Lab Chip. 3, 297–301 (2003).
S.G. Charati and S.A. Stern, Diffusion of gases in silicone polymers: Molecular dynamics simulations. Macromol. 31, 5529–5535 (1998).
M. Csete, Oxygen in the cultivation of stem cells. Ann. N. Y. Acad. Sci. 1049, 1–8 (2005).
R.R. Deshpande and E. Heinzle, On-line oxygen uptake rate and culture viability measurement of animal cell culture using microplates with integrated oxygen sensors. Biotechnol. Lett. 26, 763–767 (2004).
J.W. Dobrucki, Interaction of oxygen-sensitive luminescent probes Ru(phen)(3)(2+) and Ru(bipy)(3)(2+) with animal and plant cells in vitro. Mechanism of phototoxicity and conditions for non-invasive oxygen measurements. J. Photochem. Photobiol. B. 65, 136–144 (2001).
D.C. Duffy, J.C. McDonald, Schueller OJA, and G.M. Whitesides, Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 70, 4974–4984 (1998).
B.R. Duling, Microvascular responses to alterations in oxygen tension. Circ. Res. 31, 481–489 (1972).
T. Ezashi, P. Das, and R.M. Roberts, Low O2 tensions and the prevention of differentiation of hES cells. Proc. Natl. Acad. Sci. U S A 102, 4783–4788 (2005).
R.J. Fleischaker and A.J. Sinskey, Oxygen demand and supply in cell culture. Appl. Microbiol. Biotechnol. 12, 193 (1981).
N. Futai, W. Gu, and S. Takayama, Rapid prototyping of microstructures with bell-shaped cross-sections and its application to deformation-based microfluidic valves. Adv. Mater. 16, 1320–1323 (2004).
N. Futai, W. Gu, J.W. Song, and S. Takayama, Handheld recirculation system and customized media for microfluidic cell culture. Lab Chip. 6, 149–154 (2006).
H.C. Gerritsen, R. Sanders, A. Draaijer, and Y.K. Levine, Flourescence lifetime imaging of oxygen in living Cells. J. Flourescence. 7, 11–16 (1997).
W. Gu, X. Zhu, N. Futai, B.S. Cho, and S. Takayama, Computerized microfluidic cell culture using elastomeric channels and Braille displays. Proc. Natl. Acad. Sci. USA 101, 15861–15866 (2004).
Y.S. Heo, L.M. Cabrera, J.W. Song, N. Futai, Y-C Tung, G.D. Smith, and S. Takayama, Characterization and resolution of evaporation-mediated osmolality shifts that constrain microfluidic cell culture in poly(dimethylsiloxane) devices. Submitted to Anal. Chem. (2006).
E.Y. Hwang, D. Pappas, A.S. Jeevarajan, and M.M. Anderson, Evaluation of the paratrend multi-analyte sensor for potential utilization in long-duration automated cell culture monitoring. Biomed. Microdevices. 6, 241–249 (2004).
G.T. John, I. Klimant, C. Wittmann, and E. Heinzle, Integrated optical sensing of dissolved oxygen in microtiter plates: a novel tool for microbial cultivation. Biotechnol. Bioeng. 81, 829–836 (2003).
E. Leclerc, B. David, L. Griscom, B. Lepioufle, T. Fujii, P. Layrolle, and C. Legallaisa, Study of osteoblastic cells in a microfluidic environment. Biomaterials. 27, 586–595 (2006).
E. Leclerc, Y. Sakai, and T. Fujii, Cell culture in 3-Dimensional microfluidic structure of PDMS (polydimethylsiloxane). Biomed. Microdevices. 5, 109 (2003).
E. Leclerc, Y. Sakai, and T. Fujii, Microfluidic PDMS (polydimethylsiloxane) bioreactor for large-scale culture of hepatocytes. Biotechnol. Prog. 20, 750–755 (2004).
J. Malda, J. Rouwkema, D.E. Martens, E.P. Le Comte, F.K. Kooy, J. Tramper, C.A. van Blitterswijk, and J. Riesle, Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: measurement and modeling. Biotechnol. Bioeng. 86, 9–18 (2004).
K. Mehta and J.J. Linderman, Model-based analysis and design of a microchannel reactor for tissue engineering. Biotechnol. Bioeng. 94, 596–609 (2006).
T.C. Merkel, V.I. Bondar, K. Nagai, B.D. Freeman, and I. Pinnau, Gas sorption, diffusion, and permeation in poly(dimethylsiloxane). J. Polym. Sci. Part B-Polym. Phys. 38, 415–434 (2000).
S.M. Mitrovski and R.G. Nuzzo, An electrochemically driven poly(dimethylsiloxane) microfluidic actuator: oxygen sensing and programmable flows and pH gradients. Lab Chip. 5, 634–645 (2005).
G.F. Muschler, C. Nakamoto, and L.G. Griffith, Engineering principles of clinical cell-based tissue engineering. J. Bone Joint Surg. Am. 86-A, 1541–1558 (2004).
J. Park, T. Bansal, M. Pinelis, and M.M. Maharbiz, A microsystem for sensing and patterning oxidative microgradients during cell culture. Lab Chip. 6, 611–622 (2006).
T.H. Park and M.L. Shuler, Integration of cell culture and microfabrication technology. Biotechnol. Prog. 19, 243–253 (2003).
J.C. Pfau, J.C. Schneider, A.J. Archer, J. Sentissi, F.J. Leyva, and J. Cramton, Environmental oxygen tension affects phenotype in cultured bone marrow-derived macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L354–L362 (2004).
R.N. Pittman and B.R. Duling, Measurement of percent oxyhemoglobin in the microvasculature. J. Appl. Physiol. 38, 321–327 (1975).
P. Roy, H. Baskaran, A.W. Tilles, M.L. Yarmush, and M. Toner, Analysis of oxygen transport to hepatocytes in a flat-plate microchannel bioreactor. Ann. Biomed. Eng. 29, 947–955 (2001).
A. Seiyama, S. Tanaka, H. Kosaka, and T. Shiga, O2 transfer from single microvessels to acinar cells in secretin-stimulated pancreas of rat. Am. J. Physiol. 270, H1704–H1711 (1996).
H. Shiku, T. Saito, C-C Wu, T. Yasukawa, M. Yokoo, H. Abe, T. Matsue, and H. Yamada, Oxygen permeability of surface-modified poly(dimethylsiloxane) characterized by scanning electrochemical microscopy. Chem. Lett. 35, 234 (2006).
A. Sin, K.C. Chin, M.F. Jamil, Y. Kostov, G. Rao, and M.L. Shuler, The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotechnol. Prog. 20, 338–345 (2004).
J.W. Song, W. Gu, N. Futai, K.A. Warner, J.E. Nor, and S. Takayama, Computer-controlled microcirculatory support system for endothelial cell culture and shearing. Anal. Chem. 77, 3993–3999 (2005).
D. Sud, G. Mehta, K. Mehta, J. Linderman, S. Takayama, and M.-A. Mycek, Optical imaging in microfluidic bioreactors enables oxygen monitoring for continuous cell culture, J. Biomed. Opt. 11(5), 050504 (Published on the web on Sep. 28) (2006a).
D. Sud, W. Zhong, D.G. Beer, and M.A. Mycek, Time-resolved optical imaging provides a molecular snapshot of altered metabolic function in living human cancer cell models. Opt. Express. 14, 4412–4426 (2006b).
D.P. Swain and R.N. Pittman, Oxygen exchange in the microcirculation of hamster retractor muscle. Am. J. Physiol. 256, H247–H255 (1989).
I.R. Sweet, G. Khalil, A.R. Wallen, M. Steedman, K.A. Schenkman, J.A. Reems, S.E. Kahn, and J.B. Callis, Continuous measurement of oxygen consumption by pancreatic islets. Diabetes Technol. Ther. 4, 661–672 (2002).
N. Szita, P. Boccazzi, Z. Zhang, P. Boyle, A.J. Sinskey, and K.F. Jensen, Development of a multiplexed microbioreactor system for high-throughput bioprocessing. Lab Chip. 5, 819–826 (2005).
L. Tolosa, Y. Kostov, P. Harms, and G. Rao, Noninvasive measurement of dissolved oxygen in shake flasks. Biotechnol. Bioeng. 80, 594–597 (2002).
A.G. Tsai, P.C. Johnson, and M. Intaglietta, Oxygen gradients in the microcirculation. Physiol. Rev. 83, 933–963 (2003).
P. Urayama, W. Zhong, J.A. Beamish, F.K. Minn, R.D. Sloboda, K.H. Dragnev, E. Dmitrovsky, and M.A. Mycek, A UV-Visible-NIR fluorescence lifetime imaging microscope for laser-based biological sensing with picosecond resolution. Appl. Phys. B: Lasers and Optics. 76, 483 (2003).
V. Van Merris, M. Lenjou, D. Hoeben, G. Nijs, D. Van Bockstaele, and C. Burvenich, Culture of bovine bone marrow progenitor cells in vitro. Vet. Q. 23, 170–175 (2001).
A.P. Vollmer, R.F. Probstein, R. Gilbert, and T. Thorsen, Development of an integrated microfluidic platform for dynamic oxygen sensing and delivery in a flowing medium. Lab Chip. 5, 1059–1066 (2005).
G.M. Walker, M.S. Ozers, and D.J. Beebe, Insect cell culture in microfluidic channels. Biomed. Microdevices. 4, 161 (2002).
Y.S. Yeh, W.J. James, and H. Yasuda, Polymerization of para-xylylene derivatives.6. Morphology of parylene-N and parylene-C films investigated by gas-transport characteristics. J. Polym. Sci. Part B-Polym. Phys. 28, 545–568 (1990).
Z. Yun, Q. Lin, and A.J. Giaccia, Adaptive myogenesis under hypoxia. Mol. Cell. Biol. 25, 3040–3055 (2005).
A. Zanzotto, N. Szita, P. Boccazzi, P. Lessard, A.J. Sinskey, and K.F. Jensen, Membrane-aerated microbioreactor for high-throughput bioprocessing. Biotechnol. Bioeng. 87, 243–254 (2004).
W. Zhong, P. Urayama, M-A Mycek, Imaging fluorescence lifetime modulation of a ruthenium-based dye in living cells: the potential for oxygen sensing. J. Phys. D: Appl. Phys. 36, 1689 (2003).
Acknowledgments
We thank Dr. Brian Johnson and Prof. Mark Burns, Department of Chemical Engineering, Univ. of Michigan for use of clean room facilities, Kenneth Chomistek, Department of Chemical Engineering, Univ. of Michigan for parylene coating on PDMS. This material is based upon work supported by the U.S. Army Research Laboratory and the U.S. Army Research Office under contract/grant number DAAD19-03-1-0168 and the National Science Foundation (BES-0238625).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mehta, G., Mehta, K., Sud, D. et al. Quantitative measurement and control of oxygen levels in microfluidic poly(dimethylsiloxane) bioreactors during cell culture. Biomed Microdevices 9, 123–134 (2007). https://doi.org/10.1007/s10544-006-9005-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-006-9005-7