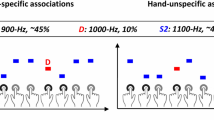
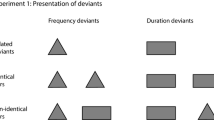
Abstract
Repetitious patterns enable the auditory system to form prediction models specifying the most likely characteristics of subsequent sounds. Pattern deviations elicit mismatch negativity (MMN), the amplitude of which is modulated by the size of the deviation and confidence in the model. Todd et al. (Neuropsychologia 49:3399–3405, 2011; J Neurophysiol 109:99–105, 2013) demonstrated that a multi-timescale sequence reveals a bias that profoundly distorts the impact of local sound statistics on the MMN amplitude. Two sounds alternate roles as repetitious “standard” and rare “deviant” rapidly (every 0.8 min) or slowly (every 2.4 min). The bias manifests as larger MMN to the sound first encountered as deviant in slow compared to fast changing sequences, but no difference for the sound first encountered as a standard. We propose that the bias is due to how Bayesian priors shape filters of sound relevance. By examining the time-course of change in MMN amplitude we show that the bias manifests immediately after roles change but rapidly disappears thereafter. The bias was reflected in the response to deviant sounds only (not in response to standards), consistent with precision estimates extracted from second order patterns modulating gain differentially for the two sounds. Evoked responses to deviants suggest that pattern extraction and reactivation of priors can operate over tens of minutes or longer. Both MMN and deviant responses establish that: (1) priors are defined by the most proximally encountered probability distribution when one exists but; (2) when no prior exists, one is instantiated by sequence onset characteristics; and (3) priors require context interruption to be updated.




Similar content being viewed by others
References
Alink A, Schwiedrzik CM, Kohler A, Singer W, Muckli L (2010) Stimulus predictability reduces responses in primary visual cortex. J Neurosci 30:2960–2966
Ayala YA, Malmierca MS (2013) Stimulus-specific adaptation and deviance detection in the inferior colliculus. Front Neural Circuits 6:89
Baldeweg T (2006) Repetition effects to sounds: evidence for predictive coding in the auditory system. Trends Cogn Sci 10:93–94
Bendixen A, Roeber U, Schröger E (2007) Regularity extraction and application in dynamic auditory stimulus sequences. J Cogn Neurosci 19:1664–1677
Butler PD, Chen Y, Ford JM, Geyer MA, Silverstein SM, Green MF (2012) Perceptual measurement in Schizophrenia: promising electrophysiology and neuroimaging paradigms from CNTRICS. Schizophr Bull 38:81–91
Cohen J (1992) A power primer. Psychol Bull 112:155–159
Costa-Faidella J, Grimm S, Slabu L, Diaz-Santaella F, Escera C (2011) Multiple time scales of adaptation in the auditory system as revealed by human evoked potentials. Psychophysiology 48:774–783
Cowan N (1984) On short and long auditory stores. Psychol Bull 96(341):370
Cowan N, Winkler I, Teder W, Näätänen R (1993) Memory prerequisites of mismatch negativity in the auditory event-related potential (ERP). J Exp Psychol-Learn Mem Cogn 19:909–921
den Ouden HE, Daunizeau J, Roiser J, Friston KJ, Stephan KE (2010) Striatal prediction error modulates cortical coupling. J Neurosci 30:3210–3219
Escera C, Alho K, Schröger E, Winkler I (2000) Involuntary attention and distractibility as evaluated with event-related brain potentials. Audiol Neurotol 5:151–166
Friston K (2005) A theory of cortical responses. Phil Trans R Soc B 360:815–836
Garrido MI, Kilner JM, Kiebel SJ, Friston K (2009) Dynamic causal modelling of the response to frequency deviants. J Neurophysiol 101:2620–2631
Griffiths TL, Kemp C, Tenenbaum JB (2008) Bayesian models of cognition. Cambridge University Press, Cambridge
Hahne A, Schröger E, Friederici AD (2002) Segregating early physical and syntactic processes in auditory sentence comprehension. NeuroReport 13:305–309
Kiebel SJ, Daunizeau J, Friston K (2008) A hierarchy of time-scales and the brain. PLoS Comput Biol 4:e1000209
Korzyukov O, Winkler I, Gumenyuk V, Alho K, Näätänen R (2003) Processing abstract auditory features in the human auditory cortex. Neuroimage 20:2245–2258
Kujala T, Tervaniemi M, Schröger E (2007) The mismatch negativity in cognitive and clinical neuroscience: theoretical and methodological considerations. Biol Psychol 74:1–19
Lieder F, Daunizeau J, Garrido MI, Friston KJ, Stephan KE (2013) Modelling trial-by-trial changes in the mismatch negativity. PLoS Comput Biol 9:e1002911. doi:10.1371/journal.pcbi.1002911
Mathys C, Jean Daunizeau J, Karl J, Friston KJ, Stephan KE (2011) A Bayesian foundation for individual learning under uncertainty Front. Hum Neurosci 5:35. doi:10.3389/fnhum.2011.00039
Näätänen R (1990) The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behav Brain Sci 13:201–288
Näätänen R (1992) Attention and brain function. Lawrence Erlbaum Associates Inc, Mahwah
Näätänen R, Alho K (1997) Mismatch negativity—the measure for central sound representation accuracy. Audiol Neurotol 2:341–353
Näätänen R, Winkler I (1999) The concept of auditory stimulus representation in cognitive neuroscience. Psychol Bull 125:826–859
Näätänen R, Gaillard AW, Mäntysalo S (1978) Early selective-attention effect on evoked potential reinterpreted. Acta Psychol (Amst) 42:313–329
Näätänen R, Paavilainen P, Alho K, Reinikainen K, Sams M (1987) The mismatch negativity to intensity changes in an auditory stimulus sequence. Electroencephalogr Clin Neurophysiol 40:125–131
Näätänen R, Kujala T, Winkler I (2011) Auditory processing that leads to conscious perception: a unique window to central auditory processing opened by the mismatch negativity and related responses. Psychophysiology 48:4–22
Pulvermüller F, Shtyrov Y, Hasting AS, Carlyon RP (2008) Syntax as a reflex: neurophysiological evidence for early automaticity of grammatical processing. Brain Lang 104:244–253
Richter N, Schröger E, Rübsamen R (2009) Hemispheric specialization during discrimination of sound sources reflected by MMN. Neuropsychologia 47:2652–2659
Ritter W, Gomes H, Cowan N, Sussman E, Vaughan HG Jr (1998) Reactivation of a dormant representation of an auditory stimulus feature. J Cogn Neurosci 10:605–614
Ritter W, Sussman E, Molholm S, Foxe JJ (2002) Memory reactivation or reinstatement and the mismatch negativity. Psychophysiology 39:158–165
Ruhnau P, Herrmann B, Schröger E (2012) Finding the right control: the mismatch negativity under investigation. Clin Neurophysiol 123:507–512
Sams M, Alho K, Näätänen R (1983) Sequential effects on the ERP in discriminating two stimuli. Biol Psychol 17:41–58
Sams M, Hari R, Rif J, Knuutila J (1993) The human auditory sensory memory trace persists about 10 sec: neuromagnetic evidence. J Cogn Neurosci 5:363–370
Schröger E (1997) On the detection of auditory deviations: a pre-attentive activation model. Int J Psychophysiol 34:245–257
Schröger E, Winkler I (1995) Presentation rate and magnitude of stimulus deviance effects on pre-attentive change detection. Neurosci Lett 193:185–188
Slabu LM, Escera C, Grimm S, Costa-Faidella J (2010) Early change detection in humans as revealed by auditory brainstem and middle-latency evoked potentials. Eur J Neurosci 32:859–865
Summerfield C, Trittschuh EH, Monti JM, Mesulam MM, Egner T (2008) Neural repetition suppression reflects fulfilled perceptual expectations. Nat Neurosci 11:1004–1006
Sussman E, Sheridan K, Kreuzer J, Winkler I (2003) Representation of the standard: stimulus context effects on the process generating the mismatch negativity component of event-related brain potentials. Int J Psychophysiol 40:465–471
Todd J, Provost A, Cooper G (2011) Lasting first impressions: a conservative bias in automatic filters of the acoustic environment. Neuropsychologia 49:3399–3405
Todd J, Michie PT, Schall U, Ward P, Catts S (2012) Mismatch negativity (MMN) reduction in schizophrenia: impaired prediction-error generation, estimation or salience? Int J Psychophysiol 83:222–231
Todd J, Provost A, Whitson L, Cooper G, Heathcote A (2013) Not so primitive: context sensitive meta-learning about unattended sound sequences. J Neurophysiol 109:99–105
Todorovic A, de Lange FP (2012) Repetition suppression and expectation suppression are dissociable in time in early auditory evoked fields. J Neurosci 32:13389–13395
Todorovic A, vanEde F, Maris E, deLange FP (2011) Prior expectation mediates neural adaptation to repeated sounds in the auditory cortex: an MEG study. J Neurosci 31:9118–9123
Ulanovsky N, Las L, Nelken I (2003) Processing of low-probability sounds by cortical neurons. Nat Neurosci 6:391–398
Wacongne C, Labyt E, van Wassenhove V, Bekinschtein T, Naccache L, Dehaene S (2011) Evidence for a hierarchy of predictions and prediction errors in human cortex. Proc Natl Acad Sci USA 108:20754–20759
Wacongne C, Changeux JP, Dehaene S (2012) A neuronal model of predictive coding accounting for the mismatch negativity. J Neurosci 32:3665–3678. doi:10.1523/JNEUROSCI.5003-11.2012
Winkler I (2007) Interpreting the mismatch negativity. Int J Psychophysiol 21:147–163
Winkler I (2010) In search for auditory object representations. In: Czigler I, Winkler I (eds) Unconscious memory representations in perception: processes and mechanisms in the brain. Advances in consciousness research. John Benjamins Publishing Company, Philadelphia, pp 71–106
Winkler I, Cowan N (2005) From sensory to long term memory: evidence from auditory memory reactivation studies. J Exp Psychol 52:3–20
Winkler I, Czigler I (1998) Mismatch negativity: deviance detection or the maintenance of the ‘standard’. NeuroReport 9:3809–3813
Winkler I, Czigler I (2012) Evidence from auditory and visual event-related potential (ERP) studies of deviance detection (MMN and vMMN) linking predictive coding theories and perceptual object representations. Int J Psychophysiol 83:132–143
Winkler I, Karmos G, Näätänen R (1996) Adaptive modelling of the unattended acoustic environment reflected in the mismatch negativity event-related potential. Brain Res 742:239–252
Winkler I, Korzyukov O, Gumenyuk V, Cowan N, Linkenkaer-Hansen K, Alho K, Ilmoniemi RJ, Näätänen R (2002) Temporary and longer retention of acoustic information. Psychophysiol 39:530–534
Acknowledgments
This research was supported by a Project Grant 1002995 from the National Health and Medical Research Council of Australia. István Winkler was supported by the Hungarian Academy of Sciences (“Lendület” LP2012-36/2012) and Andrew Heathcote by an Australian Research Council Professorial Fellowship. We offer special thanks to Gavin Cooper for programming support in recoding original sequences.
Author information
Authors and Affiliations
Corresponding author
Additional information
This is one of several papers published together in Brain Topography in the “Special Issue: Mismatch Negativity”.
Rights and permissions
About this article
Cite this article
Todd, J., Heathcote, A., Mullens, D. et al. What controls gain in gain control? Mismatch negativity (MMN), priors and system biases. Brain Topogr 27, 578–589 (2014). https://doi.org/10.1007/s10548-013-0344-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-013-0344-4