Abstract
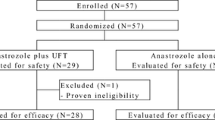
NCIC CTG MA.14 and NSABP B-29 trials examined the addition of Octreotide LAR (OCT) to 5 years of tamoxifen (TAM). Gallbladder toxicity led to B-29 discontinuation of OCT, and MA.14 OCT administration shortened to 2 years. Median follow-up was 9.8 years for 667 MA.14 patients and 6.8 years for 893 B-29 patients. The primary endpoint was disease-free survival (DFS), defined as time from randomization to time of breast cancer recurrence; second primary cancer other than squamous or basal cell skin carcinoma, cervical carcinoma in situ, or lobular breast carcinoma in situ; or death. The primary statistical test was a univariable pooled stratified log-rank test; multivariable assessment was with Cox regression. For MA.14, 97 % of patients were ≥50 years; for B-29, 62 %. MA.14 patients were 53 % lymph node negative (LN−) while B-29 were 100 % LN−; 33 % of MA.14 patients received adjuvant chemotherapy, 2 % concurrently, while B-29 had 53 % concurrent chemotherapy. MA.14 patients were 90% hormone receptor positive; B-29, 100 %. MA.14 patients experienced 5-year DFS of 80 % with TAM, 76 % with TAM + OCT; B-29 patients had 5-year DFS of 88 % for both arms. Pooled univariable TAM + OCT to TAM hazard ratio (HR) was 0.99 (95% CI 0.81–1.20; p = 0.69): for MA.14, HR = 0.94 (0.73–1.20; p = 0.50); for B-29, HR = 1.09 (0.80–1.50; p = 0.59). Multivariable pooled HR = 0.98 (0.81–1.20; p = 0.84). Older patients (p < 0.001), with higher T stage (p < 0.001), and LN + (p < 0.001) had shorter DFS. Addition of OCT to TAM did not significantly improve DFS; gallbladder toxicity shortened the additional administration of OCT. This does not negate targeting the insulin–IGF-I receptor family with less toxic therapeutics.




Similar content being viewed by others
References
Pollak M, Polychronakos C, Guyda H (1989) Somatostatin analogue SMS 201-995 reduces serum IGF-I levels in patients with neoplasms potentially dependent on IGF-I. Anticancer Res 9:889–892
van Eijck CH, Krenning EP, Bootsma A et al (1994) Somatostatin-receptor scintigraphy in primary breast cancer. Lancet 343(8898):640–643
Weckbecker G, Tolcsvai L, Stolz B et al (1994) Somatostatin analogue octreotide enhances the antineoplastic effects of tamoxifen and ovariectomy on 7, 12-dimethylbenz(a)anthracene-induced rat mammary carcinomas. Cancer Res 54(24):6334–6337
Pritchard KI, Shepherd LE, Chapman JW et al (2011) A randomized trial of tamoxifen versus combined tamoxifen and octreotide lar therapy in the adjuvant treatment of early stage breast cancer in post-menopausal women: NCIC CTG MA.14. J Clin Oncol 29(27):3869–3876
Pollak MN, Chapman JAW, Pritchard KI, et al. (2010) Tamoxifen versus tamoxifen plus octreotide LAR as adjuvant therapy for early stage breast cancer in postmenopausal women: Update of NCIC CTG MA14 trial. J Clin Oncol 28 (15S, pt I): 77s (Abstract)
Goss PE, Ingle JN, Pritchard KI, et al.; for the NCIC CTG MA.27 Study Investigators (2013). Exemestane versus Anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27 A Randomized Controlled Phase III Trial. J Clin Oncol 31(11):1398–1404
Chapman JW, Meng D, Shepherd L et al (2008) Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst 100(4):252–260
Chapman JW, Pritchard KI, Goss PE et al (2014) Competing risks of death in younger and older postmenopausal breast cancer patients. World J Clin Oncol 5(5):1088–1096
Cuzick J (2008) Primary endpoints for randomized trials of cancer therapy. Lancet Oncol 371(9631):2156–2158
Pollak M (2012) Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov 2(9):778–790
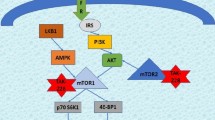
Yee D (2015) A tale of two receptors: insulin and insulin-like growth factor signaling in cancer. Clin Cancer Res 21(4):667–669
Acknowledgements
This work was supported for NCIC Clinical Trials Group MA.14 (NCT00002864) by the Canadian Cancer Society through the Canadian Cancer Society Research Institute (Grant Number 16512 to MNP), a grant from Novartis Pharmaceuticals Canada (MNP), and a NCIC Clinical Trials Group postdoctoral fellowship (BD). The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-29 (NCT00002967) was supported by the United States National Cancer Institute, Department of Health and Human Services Grants (Grant Numbers U10-CA-12027, U10-CA-69651, U10-CA-37377, U10-CA-69974, U10-CA180868, UG1-CA189867, U10-CA180822, U24-CA196067, and U24-CA114732).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Kathleen I Pritchard has declared conflict of interest with AstraZeneca, Pfizer, Roche, Amgen, Novartis, GlaxoSmithKline, and Eisai. Dr. Karen A. Gelmon has declared conflict of interest with Novartis, AstraZeneca, Pfizer, and Roche. No other authors have declared any conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chapman, JA.W., Costantino, J.P., Dong, B. et al. Octreotide LAR and tamoxifen versus tamoxifen in phase III randomize early breast cancer trials: NCIC CTG MA.14 and NSABP B-29. Breast Cancer Res Treat 153, 353–360 (2015). https://doi.org/10.1007/s10549-015-3547-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3547-4