Abstract
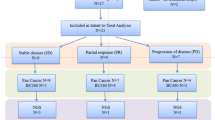
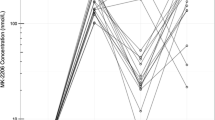
Trabectedin is an alkylating agent that binds to the minor groove of DNA. Early studies with trabectedin suggested efficacy in triple-negative and HER2-overexpressing metastatic breast cancer (MBC). The efficacy and safety of trabectedin in pretreated patients with these tumors were evaluated in this parallel-cohort phase II trial. Patients received a 3-h infusion of trabectedin 1.3 mg/m2 intravenously every 3 weeks until progression or unmanageable/unacceptable toxicity. The primary objective was to evaluate the efficacy using the objective response rate (ORR) as per Response Evaluation Criteria In Solid Tumors (RECIST). Secondary objectives comprised time-to-event endpoints and safety assessed with the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v.3.0. Patients with heavily pretreated triple-negative (n = 50) or HER2-overexpressing (n = 37) MBC were enrolled. No confirmed responses were found in triple-negative MBC patients, with median progression-free survival (PFS) of 2.2 months (95 % CI 1.3–2.7 months). Confirmed partial responses occurred in 4 of 34 evaluable HER2-overexpressing MBC patients (ORR = 12 %; 95 % CI 3–27 %) and lasted a median of 12.5 months (95 % CI, 6.2–14.7 months); median PFS was 3.8 months (95 % CI, 1.8–5.5 months). Most trabectedin-related adverse events were mild or moderate, and the most frequent were fatigue, nausea, vomiting, constipation, and anorexia. Severe neutropenia and transaminase increases were non-cumulative and transient and were mostly managed by infusion delays or dose reductions. Single-agent trabectedin is well tolerated in aggressive MBC and has moderate activity in HER2-overexpressing tumors. Further studies are warranted to evaluate trabectedin combined with HER2-targeted treatments in this subtype.


Similar content being viewed by others
References
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. doi:10.1038/35021093
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lonning PE, Borresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874. doi:10.1073/pnas.191367098
Aristei C, Leonardi C, Stracci F, Palumbo I, Luini A, Viale G, Cristallini EG, Cavaliere A, Orecchia R (2011) Risk factors for relapse after conservative treatment in T1–T2 breast cancer with one to three positive axillary nodes: results of an observational study. Ann Oncol 22(4):842–847. doi:10.1093/annonc/mdq470
Ross JS, Fletcher JA, Linette GP, Stec J, Clark E, Ayers M, Symmans WF, Pusztai L, Bloom KJ (2003) The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist 8(4):307–325
Kast K, Link T, Friedrich K, Petzold A, Niedostatek A, Schoffer O, Werner C, Klug SJ, Werner A, Gatzweiler A, Richter B, Baretton G, Wimberger P (2015) Impact of breast cancer subtypes and patterns of metastasis on outcome. Breast Cancer Res Treat 150(3):621–629. doi:10.1007/s10549-015-3341-3
Minuzzo M, Marchini S, Broggini M, Faircloth G, D’Incalci M, Mantovani R (2000) Interference of transcriptional activation by the antineoplastic drug ecteinascidin-743. Proc Natl Acad Sci USA 97(12):6780–6784
Erba E, Bergamaschi D, Bassano L, Damia G, Ronzoni S, Faircloth GT, D’Incalci M (2001) Ecteinascidin-743 (ET-743), a natural marine compound, with a unique mechanism of action. Eur J Cancer 37(1):97–105
Marco E, Garcia-Nieto R, Mendieta J, Manzanares I, Cuevas C, Gago F (2002) A 3. (ET743)-DNA complex that both resembles an RNA-DNA hybrid and mimicks zinc finger-induced DNA structural distortions. J Med Chem 45(4):871–880
D’Incalci M, Zambelli A (2015) Trabectedin for the treatment of breast cancer. Expert Opin Investig Drugs (in press)
Zelek L, Yovine A, Brain E, Turpin F, Taamma A, Riofrio M, Spielmann M, Jimeno J, Misset JL (2006) A phase II study of Yondelis (trabectedin, ET-743) as a 24-h continuous intravenous infusion in pretreated advanced breast cancer. Br J Cancer 94(11):1610–1614
Goldstein LJ, Gurtler J, Del Prete SA, Tjulandin S, Semiglazov VF, Bayever E, Michiels B (2014) Trabectedin as a single-agent treatment of advanced breast cancer after anthracycline and taxane treatment: a multicenter, randomized, phase II study comparing 2 administration regimens. Clin Breast Cancer 14(6):396–404. doi:10.1016/j.clbc.2014.06.006
Takebayashi Y, Pourquier P, Zimonjic DB, Nakayama K, Emmert S, Ueda T, Urasaki Y, Kanzaki A, Akiyama SI, Popescu N, Kraemer KH, Pommier Y (2001) Antiproliferative activity of ecteinascidin 743 is dependent upon transcription-coupled nucleotide-excision repair. Nat Med 7(8):961–966
Damia G, Silvestri S, Carrassa L, Filiberti L, Faircloth GT, Liberi G, Foiani M, D’Incalci M (2001) Unique pattern of ET-743 activity in different cellular systems with defined deficiencies in DNA-repair pathways. Int J Cancer 92(4):583–588
Schöffski P, Casali PG, Taron M, van Oosterom AT, Judson I, Grosso F, Blay JY, Maki B, Tercero JC, Jimeno J, Rosell R (2006) DNA repair functionality appears to modulate the clinical outcome of advanced sarcoma patients treated with Yondelis (ET-743). J Clin Oncol 24(18S):9522
Delaloge S, Wolp-Diniz R, Byrski T, Blum JL, Goncalves A, Campone M, Lardelli P, Kahatt C, Nieto A, Cullell-Young M, Lubinski J (2014) Activity of trabectedin in germline BRCA1/2-mutated metastatic breast cancer: results of an international first-in-class phase II study. Ann Oncol 25(6):1152–1158. doi:10.1093/annonc/mdu134
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
O’Brien PC, Fleming TR (1979) A multiple testing procedure for clinical trials. Biometrics 35(3):549–556
Lan KKG, DeMets DL (1983) Discrete sequential boundaries for clinical trials. Biometrika 70(3):659–663
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V (2007) Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 109(9):1721–1728. doi:10.1002/cncr.22618
Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong YN, Blayney DW, Niland JC, Winer EP, Weeks JC (2012) Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118(22):5463–5472. doi:10.1002/cncr.27581
Gabos Z, Thoms J, Ghosh S, Hanson J, Deschenes J, Sabri S, Abdulkarim B (2010) The association between biological subtype and locoregional recurrence in newly diagnosed breast cancer. Breast Cancer Res Treat 124(1):187–194. doi:10.1007/s10549-010-1135-1
Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA (2009) Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 115(2):423–428. doi:10.1007/s10549-008-0086-2
O’Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM, Bradley C (2011) Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med 364(3):205–214. doi:10.1056/NEJMoa1011418
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792. doi:10.1056/NEJM200103153441101
Olson EM, Najita JS, Sohl J, Arnaout A, Burstein HJ, Winer EP, Lin NU (2013) Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast 22(4):525–531. doi:10.1016/j.breast.2012.12.006
Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ (2012) Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat 133(3):831–841. doi:10.1007/s10549-011-1891-6
Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, Clark E, Ross G, Benyunes MC, Cortes J, Group CS (2015) Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372(8):724–734. doi:10.1056/NEJMoa1413513
Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, Velikova G, Olivo MS, He Y, Dutcus CE, Cortes J (2015) Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 33(6):594–601. doi:10.1200/JCO.2013.52.4892
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355(26):2733–2743. doi:10.1056/NEJMoa064320
von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, Maartense E, Zielinski C, Kaufmann M, Bauer W, Baumann KH, Clemens MR, Duerr R, Uleer C, Andersson M, Stein RC, Nekljudova V, Loibl S (2009) Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. J Clin Oncol 27(12):1999–2006. doi:10.1200/JCO.2008.19.6618
Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, Ellis C, Florance A, Vukelja S, Bischoff J, Baselga J, O’Shaughnessy J (2012) Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol 30(21):2585–2592. doi:10.1200/JCO.2011.35.6725
Demetri GD, Chawla SP, von Mehren M, Ritch P, Baker LH, Blay JY, Hande KR, Keohan ML, Samuels BL, Schuetze S, Lebedinsky C, Elsayed YA, Izquierdo MA, Gomez J, Park YC, Le Cesne A (2009) Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol 27(25):4188–4196
Schoffski P, Dumez H, Wolter P, Stefan C, Wozniak A, Jimeno J, Van Oosterom AT (2008) Clinical impact of trabectedin (ecteinascidin-743) in advanced/metastatic soft tissue sarcoma. Expert Opin Pharmacother 9(9):1609–1618. doi:10.1517/14656566.9.9.1609
Garcia-Carbonero R, Supko JG, Maki RG, Manola J, Ryan DP, Harmon D, Puchalski TA, Goss G, Seiden MV, Waxman A, Quigley MT, Lopez T, Sancho MA, Jimeno J, Guzman C, Demetri GD (2005) Ecteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic study. J Clin Oncol 23(24):5484–5492
Michaelson MD, Bellmunt J, Hudes GR, Goel S, Lee RJ, Kantoff PW, Stein CA, Lardelli P, Pardos I, Kahatt C, Nieto A, Cullell-Young M, Lewis NL, Smith MR (2012) Multicenter phase II study of trabectedin in patients with metastatic castration-resistant prostate cancer. Ann Oncol 23(5):1234–1240. doi:10.1093/annonc/mdr399
Massuti B, Cobo M, Camps C, Domine M, Provencio M, Alberola V, Vinolas N, Rosell R, Taron M, Gutierrez-Calderon V, Lardelli P, Alfaro V, Nieto A, Isla D (2012) Trabectedin in patients with advanced non-small-cell lung cancer (NSCLC) with XPG and/or ERCC1 overexpression and BRCA1 underexpression and pretreated with platinum. Lung Cancer 76(3):354–361. doi:10.1016/j.lungcan.2011.12.002
Gronchi A, Bui BN, Bonvalot S, Pilotti S, Ferrari S, Hohenberger P, Hohl RJ, Demetri GD, Le Cesne A, Lardelli P, Perez I, Nieto A, Tercero JC, Alfaro V, Tamborini E, Blay JY (2012) Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol 23(3):771–776. doi:10.1093/annonc/mdr265
Del Campo JM, Sessa C, Krasner CN, Vermorken JB, Colombo N, Kaye S, Gore M, Zintl P, Gomez J, Parekh T, Park YC, McMeekin S (2013) Trabectedin as single agent in relapsed advanced ovarian cancer: results from a retrospective pooled analysis of three phase II trials. Med Oncol 30(1):435. doi:10.1007/s12032-012-0435-1
Acknowledgments
This study was sponsored by Pharma Mar, S.A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Pilar Lardelli, Antonio Nieto, and Martin Cullell-Young—remuneration and stock ownership (Pharma Mar, S.A.). All other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Blum, J.L., Gonçalves, A., Efrat, N. et al. A phase II trial of trabectedin in triple-negative and HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat 155, 295–302 (2016). https://doi.org/10.1007/s10549-015-3675-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3675-x