Abstract
Background
Coffee contains many compounds, including antioxidants, which could prevent cancerogenesis, and coffee has been related with lower incidence of cancer at several sites. Tea is also rich in antioxidants, mainly polyphenols. To provide a quantitative overall estimate on the relation between coffee and tea consumption and glioma, we combined all published data, using a meta-analytic approach.
Methods
In September 2012, a bibliography search was carried out in both PubMed and Embase to identify observational studies providing quantitative estimates on the issue. Pooled estimates of the relative risks (RR) and the corresponding 95 % confidence intervals (CI) were calculated using random-effects models.
Results
Six studies (four cohort and two case–control studies) were available for meta-analysis, for a total of about 2100 cases. The summary RRs and 95 % CIs of glioma for drinkers versus non/occasional drinkers were 0.96 (95 % CI: 0.81–1.13) for coffee and 0.86 (95 % CI: 0.78–0.94) for tea, with no heterogeneity between studies. When we compared the highest versus the lowest categories of consumption, the RRs were 1.01 (95 % CI: 0.83–1.22) for coffee, 0.88 (95 % CI: 0.69–1.12) for tea, and 0.75 (95 % CI: 0.54–1.05) for coffee plus tea.
Conclusions
This meta-analysis, although based on few studies, suggests a lack of association between coffee intake and glioma risk, and a tendency, if any, to a lower risk for tea and coffee plus tea drinkers.
Similar content being viewed by others
Introduction
Gliomas are the most common primary brain tumors accounting for about 70 % of adult cases [1, 2]. They have an incidence of 5–10 cases per 100,000 [2]. The etiology of glioma is still largely uncertain [3]. Besides a genetic predisposition, ionizing radiation is a known risk factor [1]. Other potential risk factors include exposure to occupational and viral agents and, among dietary factors, exposure to N-nitroso compounds [1, 2].
Coffee has many effects on the central nervous system, mainly mediated by caffeine, which represents about 2–4 % of coffee powder compounds and easily crosses the blood–brain barrier. Also, a few tea components like theophylline and caffeine easily cross the blood–brain barrier. However, both coffee and tea contain many other compounds, including antioxidants, minerals, vitamin precursors, and phenols with potential anticancerogenic activities [4].
Besides general anticancerogenic activity of antioxidants, caffeine inhibits calcium ion increase by inositol 1,4,5-trisphosphate receptor subtype 3, the expression of which is increased in glioblastoma cells, consequently inhibiting migration of glioblastoma cells in various in vitro assays. Moreover, caffeine increases mean survival in a mouse xenograft model of glioblastoma [5]. Another potential mechanism through which coffee and tea might exert an anticancerogenic effect is that a tea component (polyphenol (−)-epigallocatechin-3-gallate) and two coffee components (kahweol and cafestol) inhibit DNA methyltransferase and reactivate methylation-silenced genes, including the DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT) [6, 7]. The higher activation state of MGMT has been proposed to protect against glioma and other cancers [1]. Moreover, the genetic polymorphism of MGMT has been related to the risk of glioma [8].
In epidemiological studies coffee has been related with reduced inflammation [9] and with lower incidence of cancer at the oropharynx [10, 11], liver [12], endometrium [13, 14], and colon in case–control [15], but not in prospective studies [16, 17]. The relation between black and green tea and cancer risk has been investigated in many case–control and cohort studies, showing inconsistent results [18].
The relation between coffee and/or tea consumption and glioma risk has been considered in three prospective studies [19–21] and one case–control study [22]. Two other studies, one prospective [23] and one case–control [24], considered only the relation between coffee consumption and brain cancer risk. The results of any single study are, however, based on a relatively low number of subjects. Three of these studies included also information on coffee plus tea consumption [19–21], including a recent, large European cohort study [21] finding an inverse relation.
Thus, to provide a quantitative overall estimate on this issue, we combined all published data on the relation of coffee or tea intake with glioma, using a meta-analytic approach.
Materials and methods
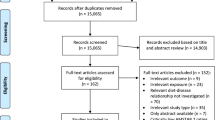
In September 2012, a bibliography search was carried out in both Medline and Embase to identify observational studies providing quantitative estimates for brain cancer risk in relation to coffee or tea consumption. The search was carried out following the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [25] and using the strings reported in the Appendix 1. The search was limited to human studies written in English. Two authors (C.P. and S.M.) independently selected the articles that reported data on the association between coffee and tea consumption and the risk of glioma or brain cancer. All case–control and cohort studies which provided estimates of the relative risk (RR) and corresponding confidence interval (CI), or information sufficient to calculate them, were included in the analyses. The reference lists of the selected papers were checked to obtain other pertinent publications. Abstracts and unpublished results were excluded. Figure 1 gives the flowchart for the selection of the articles included in the present analysis.
For each study, we extracted data on the study design, sex, country, number of cases and controls/cohort size, duration of follow-up (for cohort studies) or period of enrollment (for case–control studies), histological type of the brain tumor investigated, RR estimates for the different categories of coffee or tea intake and the corresponding 95 % CI, and, when available, the number of cases and noncases for each level of coffee or tea consumption. When more than one risk estimate was reported, we used the one adjusted for the largest number of potential confounding factors. Of the six selected papers, four considered only gliomas [19, 20, 23, 24], one considered separately gliomas and meningiomas [21], and one considered brain tumors [22]. However, of the 215 cases included in the latter study, 203 had a glioma histologic type, while the remaining 12 cases had an unspecified type of brain cancer. Thus, the analyses in the present manuscript were conducted to evaluate the relation between coffee consumption and the risk of gliomas, without considering the estimates on the risk of meningioma, since only one paper analyzed such tumor [21] and since gliomas and meningiomas are very different types of brain tumors from a pathological and clinical point of view.
Statistical analysis
Information on coffee and tea intake was collected as cups/day, except in an article reporting coffee drinking in quintiles [21]. The reference category of coffee consumption was nondrinkers in four studies [19, 20, 22, 24], occasional drinkers (<1 cup/day) in one study [23], and the lowest quintile of intake in another study [21]. The reference category of tea consumption was nondrinkers in three studies [19, 20, 22] and the lowest quintile of intake in another study [21].
The analysis comparing drinkers versus non/occasional drinkers was based on five studies for coffee intake [19, 20, 22–24] and three studies for tea intake [19, 20, 22]. One article [21] was excluded from this analysis because the risk estimates were based on quintiles of consumption, whose cutpoints were not available. To obtain the RR for “drinkers” in each study, we first pooled the RRs of each category of consumption of regular drinkers of coffee or tea within each study using the Hamling method [26], which allows us to take into account the correlation between different estimates of the same study, and then we pooled these study-specific estimates using random-effects models, which consider both within- and between-study variation.
In the analyses evaluating the highest level of coffee or tea consumption versus the lowest one, we pooled the RRs for the highest versus the lowest exposure categories of coffee or tea consumption from each study, calculating the summary estimate of the RR using random-effects models. Further, three studies analyzed total coffee plus tea consumption [19–21]. We evaluated the risk of glioma in relation to this combined exposure, as it can be considered as a proxy of total caffeine intake (approximately 90 % of all caffeine) [20]. We pooled the RRs for the highest versus the lowest exposure categories of coffee plus tea consumption from each study to calculate the summary RR using random-effects models.
Finally, we estimated the RR for the increment of coffee of one cup/day using the method proposed by Greenland and Longnecker [27], including studies with at least three categories of coffee consumption [19, 20, 23, 24]. A dose–response analysis was not conducted for tea intake, because only two studies satisfied this criterion. Before conducting a linear dose–response analysis, we checked for a nonlinear relationship between coffee consumption and the risk of glioma, by including a square term in the model. However, all the p-values for such tests were higher than 0.40.
We presented combined estimates using forest plots. In these graphs, a square was plotted for each study, whose center projection on the underlying scale corresponds to the study-specific RR and whose area is proportional to the inverse of the variance of the natural logarithm of the RR. A diamond was used to plot the summary RR, whose center represents the RR and the extremes the 95 % CIs. Statistical heterogeneity among studies was assessed using the X 2 tests (results were defined as heterogeneous for p < 0.10) [28] and was quantified using the I 2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance [29]. Usually, values of the I2 statistic <25 % are indicative of low heterogeneity, those ranging between 25 and 75 % of moderate heterogeneity, and those >75 % of high heterogeneity [29]. To evaluate publication bias, we used funnel plots [30] and Begg’s and Egger’s tests [31], where p values of the test less than 0.05 indicate the presence of publication bias.
All the analyses were performed using STATA (version 11; StataCorp, College Station, TX, USA).
Results
Figure 1 shows the flowchart for literature search and selection of articles. Review team members identified 341 articles with PubMed and 1,030 with Embase. Most of them were not focused on the relation between coffee or tea consumption and glioma/brain cancer and were therefore not considered. Of the 28 publications selected for full-text examination, 20 did not report original results from cohort or case–control studies, investigated parental coffee consumption in relation to childhood brain cancer, analyzed patients’ survival rather than cancer incidence, or analyzed patients with other specific diseases or conditions, and were therefore excluded. Eight publications were retained for the meta-analysis. Two publications [32, 33] were further excluded since they reported information on total caffeinated drinks which included also cola intake. The review of the reference lists of these publications did not identify any additional reports. Thus, the present analyses were based overall on six studies. All six studies analyzed coffee consumption (four cohort [19–21, 23] and two case–control [22, 24] studies), and four studies analyzed tea consumption (three cohort [19–21] and one case–control study [22]); three prospective studies analyzed coffee plus tea consumption [19–21] (Table 1).
Table 1 reports the main characteristics of the six studies included in the meta-analysis. Four studies were conducted in USA, one in Europe, and one in Canada. Four studies considered gliomas only, one provided separate RRs for gliomas and meningiomas, and one considered brain cancer in general. Overall, there were 2,332 cases of adult brain cancer, of which 2,075 were gliomas, 245 meningiomas (excluded from the analyses), and 12 brain cancer of unspecified histological type. Of the 1,239 cases of gliomas for which sex was specified, 768 were men and 471 women.
Figure 2 shows the RRs and 95 % CIs of glioma for coffee (Panel A) and tea (Panel B) drinkers versus non/occasional drinkers from cohort and case–control studies. The summary RR from all combined studies was 0.96 (95 % CI: 0.81–1.13, p for heterogeneity = 0.271, I 2 = 22.6 %) for the five studies analyzing coffee consumption and 0.86 (95 % CI: 0.78–0.94, p for heterogeneity = 0.419, I 2 = 0.0 %) for the three studies analyzing tea consumption. When excluding the study whose reference category was occasional drinkers [23], the summary RR for coffee consumption became 0.90 (95 % CI: 0.78–1.04, p for heterogeneity = 0.391, I 2 = 0.1 %) for drinkers verus non drinkers.
Forest plot for study-specific and pooled relative risks (RR) and 95 % confidence intervals (CI) of adult glioma for coffee (PANEL A) and tea (PANEL B) drinkers versus non/occasional drinkers. RR: relative risk, CI, confidence interval, NR, not reported, PR, persons at risk, PY, person-years. * 12 cases were brain cancers of unspecified histological type
Figure 3 shows the RRs and 95 % CIs of gliomas for the highest versus the lowest intake of coffee (Panel A), tea (Panel B), and coffee plus tea (Panel C) from cohort and case–control studies. The overall RR was 1.01 (95 % CI: 0.83–1.22, p for heterogeneity = 0.479, I2 = 0.0 %) for the six studies that analyzed coffee consumption and 0.88 (95 % CI: 0.69–1.12, p for heterogeneity = 0.208, I 2 = 34.1 %) for the four studies analyzing tea consumption. When we pooled the results of the three studies considering coffee and tea together [19–21], the overall RR for the highest versus the lowest consumption was 0.75 (95 % CI: 0.54-1.05, p for heterogeneity = 0.101, I 2 = 56.3 %).
Forest plot for study-specific and pooled relative risks (RR) and 95 % confidence intervals (CI) of adult glioma for the highest versus the lowest consumption of coffee (PANEL A), tea (PANEL B), and coffee plus tea (PANEL C). RR, relative risk, CI, confidence interval, NR, not reported, PR, persons at risk, PY, person-years. * 12 cases were brain cancers of unspecified histological type
The RR for the increment of one cup/day of coffee was 1.00 (95 % CI: 0.96–1.05, p = 0.160, I 2 = 41.9 %) for the four studies reporting three or more categories of coffee intake (Fig 4).
There was no significant asymmetry in the funnel plots, suggesting the absence of publication bias, and the tests for publication bias provided p values >0.25. However, this analysis was limited by the small number of studies.
Discussion
The present meta-analysis, based on six studies, found no association between coffee consumption and the risk of adult glioma, with consistent results between cohort and case–control studies. The finding of a slight decreased risk for tea drinkers versus non/occasional drinkers was based on three studies only and was not confirmed when the highest dose of intake was compared with the lowest, leaving the issue open to discussion. Similarly, consumption of coffee plus tea showed a tendency to a lower risk of glioma.
Among the prospective studies included in this meta-analysis, the NIH-AARP Diet and Health Study, based on 904 cases of glioma, found no relation with coffee intake up to 6 or more cups/day and a 25 % lower risk of borderline significance for drinkers of 3 or more cups/day of tea [19]. A pooled analysis of three studies (the Nurses’ Health Study I and II, and the Health Professionals Follow-Up Study) [20] found no relation of coffee or tea intake with glioma in either sex, but a RR in men of 0.46 (95 % CI: 0.26–0.81) for the highest quintile of total caffeine intake compared with the lowest one. No relation in women was found with tea intake up to ≥8 cups/week [20]. The European Prospective Investigation into Cancer and nutrition (EPIC) cohort study found no relation of glioma or meningioma with coffee or tea intake, but reported a hazard ratio of 0.66 (95 % CI: 0.45–0.96) in drinkers of >100 ml/day of coffee plus tea compared with consumers of <100 ml/day of the two beverages [21]. The Kaiser Permanente Medical Care Program of Northern California study found a slight, nonsignificant, increased risk of glioma in heavy coffee drinkers, based on 125 cases [23]. Two case–control studies were available. The first, based on 215 adult brain cancer cases (203 of which being gliomas), found no relation for ever coffee or tea drinking [22], while the second one found no relation between the risk of glioblastoma and coffee drinking up to 4 or more cups/day, as compared with nondrinkers [24].
Although based on a small number of studies, the null relation of glioma risk with consumption of coffee is likely real, as most single studies show a lack of relation, results were fairly homogeneous, the exclusion of each study in turn did not materially modify the overall risk estimate, and no significant asymmetry emerged from the funnel plot, indicating that publication bias should not have appreciably changed the results. No heterogeneity was found between studies, possibly because five of the six studies included were conducted in similar geographic areas (four in the USA and one in Canada), with similar type of coffee beverage and similar pattern of consumption. Only a multicentric study [21] was conducted in several European regions, most of which have coffee habits similar to those of northern America. However, no study reported coffee brewing methods and preparation. In the analysis to evaluate the effect of high coffee intake, the highest category included consumption higher than 4 cups/day in all studies, thus confirming the null results also for relatively high amount of coffee consumption. Moreover, the analysis for an increment of one cup/day confirmed a lack of a dose–risk relation.
A limitation inherent in meta-analyses of coffee intake is that the reference category includes some occasional drinkers. However, when we excluded the only study including occasional drinkers in the reference category, there was a tendency toward an inverse relation.
Three prospective studies [19–21] and one of the case–control studies [22] considered the relation between tea and brain cancer risk. The results of any single study were based on a relatively low number of subjects and found no relation except for a slight decreased risk in the NIH-AARP Diet and Health study [19]. However, when we pooled together the results of these studies, a significant inverse association emerged for tea consumption. Few studies were included also in the meta-analysis of tea intake, and the discrepancy in results between the analyses comparing drinkers versus non/occasional drinkers and the highest versus the lowest intake might be due to a lack of dose–risk relation, but also to the inclusion of occasional drinkers in the reference category. This might be particularly important since the highest doses of tea intake considered in two studies were relatively low, equal to ≥8 cups/week [20] and >3 cups/day [19]. Also in the EPIC study, a moderate to heavy amount of tea was consumed in four out of nine countries (the United Kingdom, followed by Denmark, the Netherland, and Germany, corresponding to about half of the subjects included in the study). In the case–control study, the determination of the absolute amount of tea intake was reported in terms of lifetime consumption [22]. We did not consider the relation between tea intake and glioma at various levels of tea intake, as only two papers considered such issue [19, 20]. They both showed no relation between tea intake at doses below 3 cups/day and a protection for higher doses, suggesting a threshold effect rather than a linear relationship. The issue is, however, still open to discussion and needs more evidence. None of the study reported the type of tea consumed (black or green). Thus, the results for tea intake are less clear, and the lack of studies conducted in Asia, where generally tea intake is much higher, leaves open the discussion on a possible inverse relation of tea intake with brain cancer risk.
Coffee plus tea intake, considered as a proxy for total caffeine intake, has been suggested to have a favorable effect on glioma risk. We found a pooled RR below unity supporting such hypothesis. However, this result was based on three studies, with only one of them showing a clear protection and thus needs to be confirmed by further research.
Two studies, excluded from the meta-analysis because they considered caffeinated beverages in general [32, 33], found a slight tendency, not statistically significant, to a decreased risk when considering the highest intake of caffeinated beverages versus the lowest, with a RR of 0.3 (95 % CI: 0.1–1.2) in the prospective study [32] and an odds ratio of 0.55 (95 % CI: 0.30–1.02) in women and 1.10 (95 % CI: 0.71–1.71) in men in the case–control study [33]. Considering that coffee and tea contribute to approximately 90 % of the total amount of caffeine consumed [20], these findings confirm the results of the present meta-analysis.
There is no explanation for the potential different effect of coffee and tea intake on adult glioma risk. A hypothesis is that substances different from methylxanthines contained mostly in tea are responsible for the favorable effect, if any, of tea intake. However, the results are still too scanty and unclear.
In conclusion, the results of this meta-analysis, although based on a small number of studies, suggest that coffee consumption is not related with the risk of adult gliomas. The analyses of the relation of glioma risk with tea or with coffee plus tea intake suggest a null relation or a moderate inverse association. However, further examination in studies conducted in populations with high tea intake is needed.
References
Ohgaki H (2009) Epidemiology of brain tumors. Methods Mol Biol 472:323–342
Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY (2012) Primary brain tumours in adults. Lancet 379:1984–1996
Schottenfeld D, Fraumeni F (2006) Cancer Epidemiology and Prevention
Yang CS, Wang X, Lu G, Picinich SC (2009) Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer 9:429–439
Kang SS, Han KS, Ku BM, Lee YK, Hong J, Shin HY, Almonte AG, Woo DH, Brat DJ, Hwang EM, Yoo SH, Chung CK, Park SH, Paek SH, Roh EJ, Lee SJ, Park JY, Traynelis SF, Lee CJ (2010) Caffeine-mediated inhibition of calcium release channel inositol 1,4,5-trisphosphate receptor subtype 3 blocks glioblastoma invasion and extends survival. Cancer Res 70:1173–1183
Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS (2003) Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res 63:7563–7570
Huber WW, Scharf G, Nagel G, Prustomersky S, Schulte-Hermann R, Kaina B (2003) Coffee and its chemopreventive components Kahweol and Cafestol increase the activity of O6-methylguanine-DNA methyltransferase in rat liver–comparison with phase II xenobiotic metabolism. Mutat Res 522:57–68
Liu Y, Shete S, Hosking FJ, Robertson LB, Bondy ML, Houlston RS (2010) New insights into susceptibility to glioma. Arch Neurol 67:275–278
Andersen LF, Jacobs DR Jr, Carlsen MH, Blomhoff R (2006) Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women’s Health Study. Am J Clin Nutr 83:1039–1046
Galeone C, Tavani A, Pelucchi C, Turati F, Winn DM, Levi F, Yu GP, Morgenstern H, Kelsey K, Dal Maso L, Purdue MP, McClean M, Talamini R, Hayes RB, Franceschi S, Schantz S, Zhang ZF, Ferro G, Chuang SC, Boffetta P, La Vecchia C, Hashibe M (2010) Coffee and tea intake and risk of head and neck cancer: pooled analysis in the international head and neck cancer epidemiology consortium. Cancer Epidemiol Biomarkers Prev 19:1723–1736
Turati F, Galeone C, La Vecchia C, Garavello W, Tavani A (2011) Coffee and cancers of the upper digestive and respiratory tracts: meta-analyses of observational studies. Ann Oncol 22:536–544
Bravi F, Bosetti C, Tavani A, Bagnardi V, Gallus S, Negri E, Franceschi S, La Vecchia C (2007) Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology 46:430–435
Je Y, Giovannucci E (2012) Coffee consumption and risk of endometrial cancer: findings from a large up-to-date meta-analysis. Int J Cancer 131:1700–1710
Bravi F, Scotti L, Bosetti C, Gallus S, Negri E, La Vecchia C, Tavani A (2009) Coffee drinking and endometrial cancer risk: a metaanalysis of observational studies. Am J Obstet Gynecol 200:130–135
Galeone C, Turati F, La Vecchia C, Tavani A (2010) Coffee consumption and risk of colorectal cancer: a meta-analysis of case-control studies. Cancer Causes Control 21:1949–1959
Je Y, Liu W, Giovannucci E (2009) Coffee consumption and risk of colorectal cancer: a systematic review and meta-analysis of prospective cohort studies. Int J Cancer 124:1662–1668
Zhang X, Albanes D, Beeson WL, van den Brandt PA, Buring JE, Flood A, Freudenheim JL, Giovannucci EL, Goldbohm RA, Jaceldo-Siegl K, Jacobs EJ, Krogh V, Larsson SC, Marshall JR, McCullough ML, Miller AB, Robien K, Rohan TE, Schatzkin A, Sieri S, Spiegelman D, Virtamo J, Wolk A, Willett WC, Zhang SM, Smith-Warner SA (2010) Risk of colon cancer and coffee, tea, and sugar-sweetened soft drink intake: pooled analysis of prospective cohort studies. J Natl Cancer Inst 102:771–783
Yuan JM, Sun C, Butler LM (2011) Tea and cancer prevention: epidemiological studies. Pharmacol Res 64:123–135
Dubrow R, Darefsky AS, Freedman ND, Hollenbeck AR, Sinha R (2012) Coffee, tea, soda, and caffeine intake in relation to risk of adult glioma in the NIH-AARP Diet and Health Study. Cancer Causes Control 23:757–768
Holick CN, Smith SG, Giovannucci E, Michaud DS (2010) Coffee, tea, caffeine intake, and risk of adult glioma in three prospective cohort studies. Cancer Epidemiol Biomarkers Prev 19:39–47
Michaud DS, Gallo V, Schlehofer B, Tjonneland A, Olsen A, Overvad K, Dahm CC, Teucher B, Lukanova A, Boeing H, Schutze M, Trichopoulou A, Lagiou P, Kyrozis A, Sacerdote C, Krogh V, Masala G, Tumino R, Mattiello A, Bueno-de-Mesquita HB, Ros MM, Peeters PH, van Gils CH, Skeie G, Engeset D, Parr CL, Ardanaz E, Chirlaque MD, Dorronsoro M, Sanchez MJ, Arguelles M, Jakszyn P, Nilsson LM, Melin BS, Manjer J, Wirfalt E, Khaw KT, Wareham N, Allen NE, Key TJ, Romieu I, Vineis P, Riboli E (2010) Coffee and tea intake and risk of brain tumors in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am J Clin Nutr 92:1145–1150
Burch JD, Craib KJ, Choi BC, Miller AB, Risch HA, Howe GR (1987) An exploratory case-control study of brain tumors in adults. J Natl Cancer Inst 78:601–609
Efird JT, Friedman GD, Sidney S, Klatsky A, Habel LA, Udaltsova NV, Van den Eeden S, Nelson LM (2004) The risk for malignant primary adult-onset glioma in a large, multiethnic, managed-care cohort: cigarette smoking and other lifestyle behaviors. J Neurooncol 68:57–69
Hochberg F, Toniolo P, Cole P, Salcman M (1990) Nonoccupational risk indicators of glioblastoma in adults. J Neurooncol 8:55–60
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Hamling J, Lee P, Weitkunat R, Ambuhl M (2008) Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 27:954–970
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135:1301–1309
Greenland S (1987) Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9:1–30
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Thornton A, Lee P (2000) Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol 53:207–216
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Blowers L, Preston-Martin S, Mack WJ (1997) Dietary and other lifestyle factors of women with brain gliomas in Los Angeles County (California, USA). Cancer Causes Control 8:5–12
Giles GG, McNeil JJ, Donnan G, Webley C, Staples MP, Ireland PD, Hurley SF, Salzberg M (1994) Dietary factors and the risk of glioma in adults: results of a case-control study in Melbourne, Australia. Int J Cancer 59:357–362
Acknowledgments
The authors thank Mrs. I. Garimoldi for editorial assistance.
Conflict of interest
The authors declare no conflict of interest. This work was conducted with the contribution of the Italian Association for Cancer Research (AIRC), project No. 10068. CG was supported by Fondazione Umberto Veronesi. SM and FT were supported by a fellowship from the Italian Foundation for Cancer Research
Author information
Authors and Affiliations
Corresponding author
Additional information
Stefano Malerba and Carlotta Galeone contributed equally to this work and are considered co-first authors.
Appendix 1
Appendix 1
(case–control OR cohort OR epidemiolog*) AND (cancer OR carcinoma OR neoplasms OR tumor OR tumour) AND (coffee OR caffeine OR beverages OR diet OR drinking OR tea OR decaf*) AND (brain OR cerebral OR intracranial OR glioma OR meningioma OR glioblastoma OR astrocytoma OR neuroblastoma OR central nervous system OR schwannoma).
for PubMed and.
‘case control’ OR cohort OR ‘epidemiology’/exp OR epidemiology AND (coffee OR ‘decaf’/exp OR beverages OR diet OR tea OR caffeine) AND (‘acoustic neuroma’/exp OR ‘astrocytoma’/exp OR ‘chordoma’/exp OR ‘cns lymphoma’ OR ‘craniopharyngioma’/exp OR ‘glioma’/exp OR ‘schwannoma’/exp OR ‘pineal’ OR ‘rhabdoid’ OR ‘pituitary’/exp OR ‘primitive neuroectodermal’ OR ‘pnet’ OR ‘ependymoma’/exp OR ‘subependymoma’/exp OR ‘oligodendroglioma’/exp OR ‘meningioma’/exp OR ‘metastatic’ OR ‘medulloblastoma’/exp OR ‘brain tumor’/exp OR ‘intercranial tumor’ OR ‘brain tumour’/exp OR ‘intracranial tumour’ OR ‘brain neoplasms’/exp OR ‘intracranial neoplasms’ OR ‘brain cancer’/exp OR ‘intracranial cancer’) for Embase.
Rights and permissions
About this article
Cite this article
Malerba, S., Galeone, C., Pelucchi, C. et al. A meta-analysis of coffee and tea consumption and the risk of glioma in adults . Cancer Causes Control 24, 267–276 (2013). https://doi.org/10.1007/s10552-012-0126-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-012-0126-4