Abstract
Purpose
Antiplatelet therapy has been widely used for management of patients with ischaemic heart diseases or thrombotic events. Experimental studies have shown that ticlopidine and clopidogrel decreased L-type Ca2+ currents (ICaL), altered action potential (AP) duration and thence exerted negative inotropic effects. In this study we tested if ticagrelor, a non-thienopyridine agent, has any influence on contractile and electrical properties of isolated ventricular myocytes.
Methods
Cardiomyocytes were isolated from male rat hearts with an enzymatic dissociation procedure and left ventricular myocytes were used for experiments. The effects of ticagrelor (1 μM) on sarcomere shortening, ionic currents and action potentials were measured at 36 ± 1 °C.
Results
Ticagrelor significantly reduced ICaL density (~18 %, p < 0.01) of ventricular myocytes and this effect was reversible. In consistence, it also decreased sarcomere shortening of electrically stimulated cardiomyocytes (13 %, p < 0.05), while it did not change relaxation rates. Repolarizing K+ currents and AP duration were unaffected by 1 μM ticagrelor application.
Conclusions
Ticagrelor exerts a significant influence on contractile properties and membrane currents of ventricular myocytes similarly to thienopyridine agents. The impact of ticagrelor on cardiac excitation-contraction coupling elements is important, since it is widely used for the treatment of patients with heart diseases.
Similar content being viewed by others
Introduction
Anti-thrombotic drugs such as clopidogrel, prasugrel and ticagrelor act through platelet P2Y12 receptors and have been widely used for clinical therapy in patients with acute coronary syndromes. Of these, ticagrelor is the first agent in a new chemical class of non-thienopyridine antiplatelet agents termed cyclopentyltriazolo- pyrimidines which is capable of blocking the platelet P2Y12 receptor to inhibit the prothrombotic effects of ADP similarly to thienopyridines. However unlike thienopyridines, which are irreversible antagonists, it binds reversibly to the P2Y12 receptor at a distinct, noncompetitive site and does not require metabolic activation [1–3].
Transient dyspnea in association with asymptomatic bradycardia has been reported at the onset of ticagrelor therapy [3, 4]. Several mechanisms have been proposed that may account for ticagrelor-induced bradyarrhythmias including altered cardiac conduction due to direct P2Y12 inhibition. However modulation of inward-rectifier potassium currents (IK1) and funny currents (If) of nodal cells through indirect mechanisms have been also raised as the likely explanation of bradyarrhythmic events [5]. Jakubowski et al. [6] have shown that thienopyridines and their thienopyrimidinone analogues exert a negative inotropic effect additionally to coronary vasodilator properties in the isolated guinea pig heart. In fact ticlopidine and clopidogrel elicited a remarkable negative inotropic effect in the isolated guinea pig heart (dP/dtmax was suppressed by 15–30 %). Consistent with this the L-type Ca2+ current (ICaL) which plays a predominant role in Ca2+ influx for the initiation of cardiac contraction cycle and thereby regulating heart inotropy has been suggested as the likely mechanism for decreased inotropy in isolated guinea pig heart [7]. In fact, thienopyridines such as ticlopidine and clopidogrel have been shown to reduce ICaL density and shorten action potential (AP) duration in isolated guinea pig ventricular myocytes and human atrial myocytes, although the effect of the former was more pronounced [7]. However, the impact of ticagrelor on contractility and ionic mechanisms of ventricular myocytes have not been investigated yet. Accordingly, the aim of the present study was to examine whether ticagrelor influences inotropic activity and ionic currents (ICaL and K+ currents) of isolated ventricular myocytes of rat heart. Since it has been widely used for clinical therapy, potential alterations of contractile function and/or electrical activity of cardiac myocytes may be of special interest for those patients suffering from cardiac diseases associated with these cellular abnormalities.
Materials and Methods
Animals and Isolation of Ventricular Myocytes
Three-month-old male Wistar rats, weighing 250–300 g, were used in these experiments. Isolation of cardiac myocytes was performed enzymatically as described in our previous studies [8, 9]. Briefly, rats were anesthetized with pentobarbital sodium (50 mg/kg body weight, ip) and the heart was excised from the thoracic cavity quickly. Cannulation of the aorta was carried out with Langerdorff apparatus and perfused retrogradely through the coronary arteries with Ca2 + −free solution containing (in mM: 137 NaCl, 5.4 KCl, 1.2 MgSO4, 1.2 KH2PO4, 6 HEPES and 20 glucose at pH 7.2, bubbled with 100 % O2) for 5 min and then switched to the same solution to which was added 0.8 mg/ml collagenase (Collagenase A Roche) and 0.07 mg/ml protease (Sigma type XIV) for approximately 20 min. Ventricles were then removed and minced into small pieces and gently passed through a nylon mesh. Then, cell suspension was washed several times and Ca2+ levels were increased gradually for adaptation of myocytes. Experiments were started 1 h after the isolation of the ventricular cells and performed at 36 ± 1 °C.
Solutions and Chemicals
For contractility and patch clamp experiments, cells were superfused with normal Tyrode’s solution (in mM: 137 NaCl, 5.4 KCl, 0.5 MgCl2, 1.5 CaCl2, 11.8 HEPES and 10 glucose, pH adjusted to 7.40 with NaOH). The pipette solution for whole-cell patch clamp contained (in mM): 120 K-aspartate, 20 KCl, 10 NaCl, 10 K-HEPES, 5 MgATP (pH = 7.2). However for measurement of ICaL, K+ was replaced with Cs+ in the external Tyrode and in the pipette solution, and 10 mM EGTA was added to the internal solution while interference of calcium currents was prevented by cadmium chloride (250 μM) during K+ current recording. Ticagrelor (Cayman Chemicals, Ann Arbor, Michigan USA) was dissolved in DMSO where the final dilution of DMSO in experimental solutions was <0.01 %.
Electrophysiological Recordings
Sarcomere Shortening
Myocytes were superfused with tyrode solution continuously and sarcomere length was recorded using the IonOptix MyoCam system (IonOptix LLC, Milton USA) contraction during field stimulation at 0.5 Hz. At least 10 contractions of every phase were averaged for fractional sarcomere shortening (L/L0), time to peak shortening (TPS) and time to 90 % relengthening (RT90) using IonOptix software.
Membrane Currents and Action Potentials
Currents were recorded by whole-cell configuration of patch-clamp amplifier (Axon200B, Molecular Devices, USA). A voltage-clamp protocol consisting of a prepulse from −70 to −45 mV (inactivation of Na+ currents), followed by 300 ms depolarizing step to 0 mV was applied for the measurement of ICaL. The amplitude of ICaL was measured as the difference between peak current and the current at the end of the pulse. Current density was calculated by dividing the currents to cell capacitance and results were presented as pA/pF.
Action potentials were recorded in a current clamp mode and elicited by injection of 1–3 ms current at 1 Hz frequency. Total K+ currents were recorded in a voltage clamp mode using ramp protocols. The descending ramp protocol from +80 to −120 mV was applied for 2 s where the myocyte was held at −40 mV. This protocol allows us to assess the values of both transient outward (Ito) and IK1 currents. The data collected and analyzed using pCLAMP and Clampfit software (Version 10, Axon Instruments, USA) respectively.
Statistical Analysis
Data were presented as the mean ± SEM. Statistical analysis was performed with paired t-test and p values smaller than 0.05 were considered significant.
Results
Effect of Ticagrelor on L-Type Ca2+ Currents
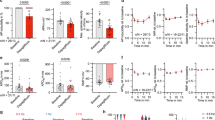
We first studied the effect of ticagrelor on Ca2+currents in rat ventricular myocytes. Figure 1 shows Ca2+ currents recorded by applying a voltage-clamp protocol consisting of a pre-pulse from −70 to −45 mV and followed by 300 ms depolarizing pulse to 0 mV in the absence and presence of 1 μM of ticagrelor. The results indicate that ticagrelor was capable of reducing Ca2+currents significantly and this effect was reversible after washout of the drug with external solution (Fig. 1a). The extent of reduction in the peak current of LTCC was ~18 % at 1 μM (n = 11) of ticagrelor concentration (Fig. 1b).
Effect of ticagrelor on ICaL. a Representative ICaL traces and peak ICaL values as a function of time in the absence and presence of ticagrelor. Currents were recorded by depolarizing pulse to 0 mV where holding potential is −40 mV to inactivate Na+ currents. b Percent change in ICaL density after wash in and washout of 1 μM ticagrelor. c Effect of ticagrelor on ICaL in the presence of adenosine receptor inhibitor theophylline (1 μM). Data are presented as mean ± SEM. N = 11, *p < 0.01 versus Control, # p < 0.05 versus ticagrelor
To elucidate the involvement of adenosine receptor activation on the effect of ticagrelor we performed experiments with theophylline (1 μM) which is a non-selective blocker of adenosine receptors. Although perfusion of ventricular myocytes with theophylline reduced Ca2+ currents to some extent, ticagrelor achieved further reduction in the presence of theophylline (Fig. 1c), which implies that adenosine receptor mediated effect is unlikely.
Effect of Ticagrelor on Ventricular Myocyte Contractility
To assess whether the decrement of ICaL leads to contractile changes we perfused the electrically stimulated ventricular myocytes (5–10 V and 0.5 Hz) with the same concentration of ticagrelor. As it can be seen from Fig. 2 ticagrelor reduced fractional shortening of ventricular myocytes by 13 % (n = 10) and washout of the drug following the response elicited almost a complete reversal of contractility. However, ticagrelor did not affect either contraction rate or relaxation rate of shortening in electrically stimulated myocytes (Fig. 2). These results indicate that ticagrelor exerts negative inotropic effects, but it didn’t change myocardial relaxation.
Effect of ticagrelor on fractional shortening of ventricular myocytes. Myocyte contraction was measured as the change in sarcomere length during stimulation of cells at 0.5 Hz frequency in the absence and presence of ticagrelor. Data are presented as mean ± SEM. N = 10, *p < 0.05 versus Control, # p < 0.05 versus ticagrelor
Effect of Ticagrelor on K+ Currents and AP Duration
To assess the effects of ticagrelor on K+ currents, voltage ramps where the myocytes were held at −40 mV and then depolarized to +80 mV, followed by a 2 s repolarizing ramp to −120 mV were applied (Fig. 3a, top record). In this protocol the initial depolarization elicited an outward current that decreased gradually following the onset of repolarization and the outward current disappeared as the membrane potential became more negative and eventually was replaced by an inward current. Previous studies have suggested that the outward current recorded under these conditions is characterized as Ito and the inward component, on the other hand, is characterized as IK1 [10, 11]. In Fig. 3a current tracings were re-plotted against the ramp voltage to generate the I–V curves which shows that ticagrelor didn’t elicit significant changes either in Ito or IK1 (N = 8).
Effects of ticagrelor on K+ currents recorded during hyperpolarising voltage ramps and on AP obtained with current clamp. a Ramp protocol from +80 to −120 mV was applied for 2 s where the myocyte was held at −40 mV. Membrane currents were re-plotted against the ramp voltage in the absence and presence of ticagrelor (N = 8). b Representative AP traces recorded before and during ticagrelor wash in and elicited by injection of 1–3 ms current at 1 Hz frequency (N = 13)
Figure 3b shows sample APs in the absence and presence of 1 μM ticagrelor where it didn’t achieve any significant change in AP duration (APD90: 48.54 ± 7.63 versus 51.420 ± 7.63 n = 13 and p > 0.05). Furthermore neither peak values nor resting membrane potential were altered by ticagrelor application (data not shown).
Discussion
Ticagrelor is a nonthienopyridine antiplatelet agent that blocks the platelet P2Y12 receptor to inhibit the prothrombotic effects of ADP. Unlike the thienopyridines, it binds reversibly to P2Y12 receptors without requiring metabolic activation [12]. Due to these eminent properties with respect to thienopyridines, which are irreversible antagonists, it has been shown to reduce platelet aggregation more effectively and to improve cardiovascular outcome in patients with acute coronary syndromes [13, 14]. Emerging evidence suggests that ticagrelor may also provide considerable clinical benefits by reducing mortality related to cardiovascular events, myocardial infarction or stroke [15]. Recently it was shown that ticagrelor increases myocardial calcium-dependent NOS activity, stimulates endothelium NO release and thereby reduces myocardial infarct size in a rat model [16]. Nevertheless, although thienopyridines have been reported to attain reduced inotropy in guinea pig heart, the effect of ticagrelor on electrical and contractile properties of myocardium has not been examined. To the extent of our knowledge, no data have been presented on the potential impact of ticagrelor on electrophysiological properties of the heart in isolated ventricular myocytes. In this study we clearly showed, for the first time, that like thienopyridines ticagrelor exerts a discernible influence on contractile properties and ion channels of ventricular myocytes.
We demonstrated that ticagrelor significantly reduced fractional shortening of ventricular myocytes and suppressed ICaL at 1 μM concentration. These results are in parallel with findings of Jakubowski et al., which showed negative inotropy and reduced ICaL density along with half maximal potential shift to more negative values in guinea pig heart with ticlopidine and clopidogrel [6, 7]. In a further step we observed that ticagrelor was capable of reducing contractility of isolated ventricular myocytes whereas it did not change contraction and relaxation rates. It is most likely that the negative inotropy elicited by ticagrelor is caused by a decrease of Ca2+ influx through L-type Ca2+ channels.
The molecular target of pharmacological agents that inhibit platelet aggregation has been described as the G-protein coupled purinoreceptors P2Y12. Although the expression of this receptor has been identified in neuronal, endothelial and vascular tissues as well as platelets, no data have been presented for cardiac myocytes until now [17–19]. Involvement of P2Y12 receptors in the modulation of ICaL in guinea pig cardiomyocytes was found unlikely [7], since ticlopidine and clopidogrel are prodrugs which have to be converted to active metabolites to exert their effects on P2Y12 receptors [15, 20]. Ticagrelor on the other hand doesn’t need hepatic metabolic activation and has unique pharmacologic properties including quick onset and quick response time [15]. Therefore we cannot exclude the presence of P2Y12 receptor-mediated action of ticagrelor on ICaL and contractility in a cell-free experimental environment. Adenosine receptor mediated effect is not the likely mechanism of reduced ICaL since the impact of ticagrelor persisted even in the presence of non-selective adenosine receptor inhibitor, theophylline. Alternatively, it was shown that both thienopyridines and ticagrelor can stimulate NO release via upregulated activation of NOS [6, 7, 16, 21] which may cause an increase in cGMP levels. Consistent with this, we recently showed decreased ICaL and reduced contractility in rat ventricular myocytes due to activation of the NOS-GC-cGMP pathway [9].
The effect of ticagrelor on AP duration and repolarizing K+ currents was also examined to assess whether it can change electrical activities of ventricular myocytes. Unlike previous findings showing AP shortening with ticlodipine [7], our measurements showed no difference either in AP duration or total K+ currents. This is interesting since ICaL is prominent for the plateau phase of action potential in ventricular cardiomyocytes. Species difference may explain these controversial results. Because the AP of rat cardiomyocytes does not involve a plateau unlike guinea pig and human myocytes and repolarization of AP is achieved predominantly by K+ currents which did not change in our experiments. Similar studies that will be performed on different species are highly essential to verify the influence of ticagrelor on contractility and ionic currents of cardiomyocytes.
In summary, ticagrelor reduces ICaL density and contractility in isolated ventricular mtocytes, while it does not alter either AP duration or repolarizing K+ currents. It is of critical importance to characterize the influence of ticagrelor and other antiplatelet agents on cardiac excitation-contraction coupling elements, since they are widely used for the treatment of patients with heart diseases. Importantly, this negative inotropic effect of ticagrelor can be the likely mechanism of dyspnea which may occur at the onset of ticagrelor therapy. However, the exact sub-cellular mechanisms by which ticagrelor exerts these effects remains to be elucidated. This study is also limited by excluding the contribution of adenosine receptors completely in the applied concentration which should have been preferable due to possible non-specific actions of theophylline at higher concentrations [22, 23]. Further experiments performed with different inhibitors specifically aimed at potential effectors as well as adenosine receptors will figure out the cellular mechanisms that may play role in ticagrelor related alterations in cardiac myocytes.
References
Van Giezen JJ, Nilsson L, Berntsson P, Wissing BM, Giordanetto F, Tomlinson W, et al. Ticagrelor binds to human P2Y(12) independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J Thromb Haemost. 2009;7(9):1556–65.
Flierl U, Schöpp C, Jaitner J, Bauersachs J, Schäfer A. The novel P2Y 12 antagonist AZD6140 rapidly and reversibly reduces platelet activation in diabetic rats. Thromb Res. 2010;125(3):e93–9.
Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006;27(9):1038–47.
Cannon CP, Husted S, Harrington RA, Scirica BM, Emanuelsson H, Peters G, et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol. 2007;50(19):1844–51.
Di Serafino L, Rotolo FL, Boggi A, Colantonio R, Serdoz R, Monti F. Potential additive effects of ticagrelor, ivabradine, and carvedilol on sinus node. Case Rep Cardiol. 2014;2014:932595.
Jakubowski A, Chlopicki S, Olszanecki R, Jawien J, Lomnicka M, Dupin JP, et al. Endothelial action of thienopyridines and thienopyrimidinones in the isolated guinea pig heart. Prostaglandins Leukot Essent Fat Acids. 2005;72(2):139–45.
Pelzmann B, Zorn-Pauly K, Hallström S, Mächler H, Jakubowski A, Lang P, et al. Effects of thienopyridines and thienopyrimidinones on L-type calcium current in isolated cardiomyocytes. Naunyn Schmiedeberg’s Arch Pharmacol. 2010;382(5–6):433–40.
Ozturk N, Yaras N, Ozmen A, Ozdemir S. Long-term administration of rosuvastatin prevents contractile and electrical remodelling of diabetic rat heart. J Bioenerg Biomembr. 2013;45(4):343–52.
Olgar Y, Ozturk N, Usta C, Puddu PE, Ozdemir S. Ellagic acid reduces L-type Ca2+ current and contractility through modulation of NO-GC-cGMP pathways in rat ventricular myocytes. J Cardiovasc Pharmacol. 2014;64(6):567–73.
Chorvatova A, Hussain M. Effects of caffeine on potassium currents in isolated rat ventricular myocytes. Pflugers Arch. 2003;446(4):422–8.
Pearman C, Kent W, Bracken N, Hussain M. H-89 inhibits transient outward and inward rectifier potassium currents in isolated rat ventricular myocytes. Br J Pharmacol. 2006;148(8):1091–8.
Abergel E, Nikolsky E. Ticagrelor: an investigational oral antiplatelet treatment for reduction of major adverse cardiac events in patients with acute coronary syndrome. Vasc Health Risk Manag. 2010;6:963–77.
Storey RF, Husted S, Harrington RA, Heptinstall S, Wilcox RG, Peters G, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007;50(19):1852–6.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Steiner JB, Wu Z, Ren J. Ticagrelor: positive, negative and misunderstood properties as a new antiplatelet agent. Clin Exp Pharmacol Physiol. 2013;40(7):398–403.
Nanhwan MK, Ling S, Kodakandla M, Nylander S, Ye Y, Birnbaum Y. Chronic treatment with ticagrelor limits myocardial infarct size: an adenosine and cyclooxygenase-2-dependent effect. Arterioscler Thromb Vasc Biol. 2014;34(9):2078–85.
Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4(1):1–20.
Burnstock G, Pelleg A. Cardiac purinergic signalling in health and disease. Purinergic Signal. 2015;11(1):1–46.
Cheung KK, Marques-da-Silva C, Vairo L, dos Santos DS, Goldenberg R, Coutinho-Silva R, et al. Pharmacological and molecular characterization of functional P2 receptors in rat embryonic cardiomyocytes. Purinergic Signal. 2015;11(1):127–38. doi:10.1007/s11302-014-9441-4.
Sharis PJ, Cannon CP, Loscalzo J. The antiplatelet effects of ticlopidine and clopidogrel. Ann Intern Med. 1998;129(5):394–405.
Kirkby NS, Lundberg MH, Chan MV, Vojnovic I, Solomon AB, Emerson M, et al. Blockade of the purinergic P2Y12 receptor greatly increases the platelet inhibitory actions of nitric oxide. Proc Natl Acad Sci U S A. 2013;110(39):15782–7.
Pierce KD, Furlong TJ, Selbie LA, Shine J. Molecular cloning and expression of an adenosine A2b receptor from human brain. Biochem Biophys Res Commun. 1992;187(1):86–93.
Sattin A, Rall TW. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3′, 5′-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970;6(1):13–23.
Acknowledgments
This work was supported by Akdeniz University Research Coordination Unit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kucuk, M., Celen, M.C., Yamasan, B.E. et al. Effects of Ticagrelor on Ionic Currents and Contractility in Rat Ventricular Myocytes. Cardiovasc Drugs Ther 29, 419–424 (2015). https://doi.org/10.1007/s10557-015-6617-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-015-6617-2