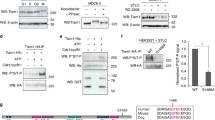
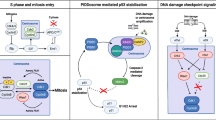
Abstract
Centrosomes are microtubule-organizing centers that duplicate in S phase to form bipolar spindles that separate duplicated chromosomes faithfully into two daughter cells during cell division. Recent studies show that proper timing of centrosome dynamics, the disjunction and movement of centrosomes, is tightly linked to spindle symmetry, correct microtubule-kinetochore attachment, and chromosome segregation. Here, we review mechanisms that regulate centrosome dynamics, with emphasis on the roles of key mitotic kinases in the proper timing of centrosome dynamics and how aberrancies in these processes may cause chromosomal instability and cancer.


Similar content being viewed by others
Abbreviations
- Cdk1:
-
Cyclin-dependent kinase 1
- Cep85:
-
Centrosome protein 85 KDa
- EGF:
-
Epidermal growth factor
- EGFR:
-
Epidermal growth factor receptor
- GRK2:
-
G protein-coupled receptor kinase 2
- Sav1:
-
Scaffold protein Salvador
- KT:
-
Kinetochore
- MEF:
-
Mouse embryonic fibroblast
- Mst2:
-
Mammalian sterile 20-like kinase 2
- NE:
-
Nuclear envelope
- NEBD:
-
Nuclear envelope breakdown
- Nek2A:
-
NIMA family kinase 2A
- NIMA:
-
Never in Mitosis A
- NPC:
-
Nuclear pore complex
- PCM:
-
Pericentriolar material
- Plk1:
-
Polo-like kinase 1
- TEIF:
-
Transcriptional element-interacting factor
References
Azimzadeh J, Bornens M (2007) Structure and duplication of the centrosome. J Cell Sci 120:2139–2142
Bahe S, Stierhof YD, Wilkinson CJ, Leiss F, Nigg EA (2005) Rootletin forms centriole-associated filaments and functions in centrosome cohesion. J Cell Biol 171:27–33
Bahmanyar S, Kaplan DD, Deluca JG, Giddings TH Jr, O’toole ET, Winey M, Salmon ED, Casey PJ, Nelson WJ, Barth AI (2008) Beta-catenin is a Nek2 substrate involved in centrosome separation. Genes Dev 22:91–105
Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW (2006) Flies without centrioles. Cell 125:1375–1386
Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J (2002) Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell 108:83–96
Belham C, Roig J, Caldwell JA, Aoyama Y, Kemp BE, Comb M, Avruch J (2003) A mitotic cascade of NIMA family kinases: Nercc1/Nek9 activates the Nek6 and Nek7 kinases. J Biol Chem 278:34897–34909
Bertran MT, Sdelci S, Regue L, Avruch J, Caelles C, Roig J (2011) Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5. EMBO J 30:2634–2647
Bettencourt-Dias M, Glover DM (2007) Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol 8:451–463
Blangy A, Lane HA, D’herin P, Harper M, Kress M, Nigg EA (1995) Phosphorylation by P34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83:1159–1169
Bornens M (2012) The centrosome in cells and organisms. Science 335:422–426
Brandeis M, Rosewell I, Carrington M, Crompton T, Jacobs MA, Kirk J, Gannon J, Hunt T (1998) Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc Natl Acad Sci U S A 95:4344–4349
Bruinsma W, Raaijmakers JA, Medema RH (2012) Switching polo-like kinase-1 on and off in time and space. Trends Biochem Sci 37:534–542
Castillo A, Morse HC 3rd, Godfrey VL, Naeem R, Justice MJ (2007) Overexpression of Eg5 causes genomic instability and tumor formation in mice. Cancer Res 67:10138–10147
Chen XJ, Levedakou EN, Millen KJ, Wollmann RL, Soliven B, Popko B (2007) Proprioceptive sensory neuropathy in mice with a mutation in the cytoplasmic dynein heavy chain 1 gene. J Neurosci 27:14515–14524
Chen C, Tian F, Lu L, Wang Y, Xiao Z, Yu C, Yu X (2015) Characterization of Cep85: a novel antagonist of Nek2a that is involved in the regulation of centrosome disjunction. J Cell Sci
Chilov D, Sinjushina N, Rita H, Taketo MM, Makela TP, Partanen J (2011) Phosphorylated beta-catenin localizes to centrosomes of neuronal progenitors and is required for cell polarity and neurogenesis in developing midbrain. Dev Biol 357:259–268
De Souza EE, Meirelles GV, Godoy BB, Perez AM, Smetana JH, Doxsey SJ, Mccomb ME, Costello CE, Whelan SA, Kobarg J (2014) Characterization of the human Nek7 interactome suggests catalytic and regulatory properties distinct from those of Nek6. J Proteome Res 13:4074–4090
Dohadwala M, Da Cruz e Silva EF, Hall FL, Williams RT, Carbonaro-Hall DA, Nairn AC, Greengard P, Berndt N (1994) Phosphorylation and inactivation of protein phosphatase 1 by cyclin-dependent kinases. Proc Natl Acad Sci U S A 91:6408–6412
Feige E, Motro B (2002) The related murine kinases, Nek6 and Nek7, display distinct patterns of expression. Mech Dev 110:219–223
Fry AM, Mayor T, Meraldi P, Stierhof YD, Tanaka K, Nigg EA (1998) C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J Cell Biol 141:1563–1574
Gayek AS, Ohi R (2014) Kinetochore-microtubule stability governs the metaphase requirement for Eg5. Mol Biol Cell 25:2051–2060
Gonczy P, Pichler S, Kirkham M, Hyman AA (1999) Cytoplasmic dynein is required for distinct aspects of Mtoc positioning, including centrosome separation, in the one cell stage caenorhabditis elegans embryo. J Cell Biol 147:135–150
Graser S, Stierhof YD, Nigg EA (2007) Cep68 and Cep215 (Cdk5rap2) are required for centrosome cohesion. J Cell Sci 120:4321–4331
Guo Y, Zheng Y (2015) Lamins position the nuclear pores and centrosomes by modulating dynein. Mol Biol Cell
Hadjihannas MV, Bruckner M, Behrens J (2010) Conductin/Axin2 and Wnt signalling regulates centrosome cohesion. EMBO Rep 11:317–324
Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, Ahmad-Annuar A, Bowen S, Lalli G, Witherden AS, Hummerich H, Nicholson S, Morgan PJ, Oozageer R, Priestley JV, Averill S, King VR, Ball S, Peters J, Toda T, Yamamoto A, Hiraoka Y, Augustin M, Korthaus D, Wattler S, Wabnitz P, Dickneite C, Lampel S, Boehme F, Peraus G, Popp A, Rudelius M, Schlegel J, Fuchs H, Hrabe De Angelis M, Schiavo G, Shima DT, Russ AP, Stumm G, Martin JE, Fisher EM (2003) Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science 300:808–812
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Hardy T, Lee M, Hames RS, Prosser SL, Cheary DM, Samant MD, Schultz F, Baxter JE, Rhee K, Fry AM (2014) Multisite phosphorylation of C-Nap1 releases it from Cep135 to trigger centrosome disjunction. J Cell Sci 127:2493–2506
He R, Huang N, Bao Y, Zhou H, Teng J, Chen J (2013) Lrrc45 is a centrosome linker component required for centrosome cohesion. Cell Rep 4:1100–1107
Hinchcliffe EH, Miller FJ, Cham M, Khodjakov A, Sluder G (2001) Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science 291:1547–1550
Hochegger H, Takeda S, Hunt T (2008) Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat Rev Mol Cell Biol 9:910–916
Huang P, Senga T, Hamaguchi M (2007) A novel role of phospho-beta-catenin in microtubule regrowth at centrosome. Oncogene 26:4357–4371
Kapitein LC, Peterman EJG, Kwok BH, Kim JH, Kapoor TM, Schmidt CF (2005) The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature 435:114–118
Kaplan DD, Meigs TE, Kelly P, Casey PJ (2004) Identification of a role for beta-catenin in the establishment of a bipolar mitotic spindle. J Biol Chem 279:10829–10832
Kardon JR, Vale RD (2009) Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol 10:854–865
Khodjakov A, Cole RW, Oakley BR, Rieder CL (2000) Centrosome-independent mitotic spindle formation in vertebrates. Curr Biol 10:59–67
Kiyomitsu T, Cheeseman IM (2012) Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation. Nat Cell Biol 14:311–317
Kotak S, Busso C, Gonczy P (2012) Cortical dynein is critical for proper spindle positioning in human cells. J Cell Biol 199:97–110
Le Guellec R, Paris J, Couturier A, Roghi C, Philippe M (1991) Cloning by differential screening of a Xenopus Cdna that encodes a kinesin-related protein. Mol Cell Biol 11:3395–3398
Lindqvist A, Rodriguez-Bravo V, Medema RH (2009) The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol
Liu D, Davydenko O, Lampson MA (2012) Polo-like kinase-1 regulates kinetochore-microtubule dynamics and spindle checkpoint silencing. J Cell Biol 198:491–499
Mardin BR, Schiebel E (2012) Breaking the ties that bind: new advances in centrosome biology. J Cell Biol 197:11–18
Mardin BR, Lange C, Baxter JE, Hardy T, Scholz SR, Fry AM, Schiebel E (2010) Components of the hippo pathway cooperate with Nek2 kinase to regulate centrosome disjunction. Nat Cell Biol 12:1166–1176
Mardin BR, Agircan FG, Lange C, Schiebel E (2011) Plk1 controls the Nek2a-Pp1gamma antagonism in centrosome disjunction. Curr Biol 21:1145–1151
Mardin BR, Isokane M, Cosenza MR, Kramer A, Ellenberg J, Fry AM, Schiebel E (2013) EGF-induced centrosome separation promotes mitotic progression and cell survival. Dev Cell 25:229–240
Marshall WF, Rosenbaum JL (1999) Cell division: the renaissance of the centriole. Curr Biol 9:R218–R220
Mayor T, Stierhof YD, Tanaka K, Fry AM, Nigg EA (2000) The centrosomal protein C-Nap1 is required for cell cycle-regulated centrosome cohesion. J Cell Biol 151:837–846
Meraldi P, Nigg EA (2001) Centrosome cohesion is regulated by a balance of kinase and phosphatase activities. J Cell Sci 114:3749–3757
Meraldi P, Nigg EA (2002) The centrosome cycle. FEBS Lett 521:9–13
Minoguchi S, Minoguchi M, Yoshimura A (2003) Differential control of the NIMA-related kinases, Nek6 and Nek7, by serum stimulation. Biochem Biophys Res Commun 301:899–906
Nam HJ, Van Deursen JM (2014) Cyclin B2 and P53 control proper timing of centrosome separation. Nat Cell Biol 16:538–549
Nigg EA (2007) Centrosome duplication: of rules and licenses. Trends Cell Biol 17:215–221
Nigg EA, Raff JW (2009) Centrioles, centrosomes, and cilia in health and disease. Cell 139:663–678
Nigg EA, Stearns T (2011) The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol 13:1154–1160
O’regan L, Fry AM (2009) The Nek6 and Nek7 protein kinases are required for robust mitotic spindle formation and cytokinesis. Mol Cell Biol 29:3975–3990
Prosser SL, Sahota NK, Pelletier L, Morrison CG, Fry AM (2015) Nek5 promotes centrosome integrity in interphase and loss of centrosome cohesion in mitosis. J Cell Biol 209:339–348
Raaijmakers JA, Tanenbaum ME, Medema RH (2013) Systematic dissection of dynein regulators in mitosis. J Cell Biol 201:201–215
Rapley J, Nicolas M, Groen A, Regue L, Bertran MT, Caelles C, Avruch J, Roig J (2008) The NIMA-family kinase Nek6 phosphorylates the kinesin Eg5 at a novel site necessary for mitotic spindle formation. J Cell Sci 121:3912–3921
Regue L, Sdelci S, Bertran MT, Caelles C, Reverter D, Roig J (2011) Dynll/Lc8 protein controls signal transduction through the Nek9/Nek6 signaling module by regulating Nek6 binding to Nek9. J Biol Chem 286:18118–18129
Robinson JT, Wojcik EJ, Sanders MA, Mcgrail M, Hays TS (1999) Cytoplasmic dynein is required for the nuclear attachment and migration of centrosomes during mitosis in drosophila. J Cell Biol 146:597–608
Roig J, Mikhailov A, Belham C, Avruch J (2002) Nercc1, a mammalian NIMA-family kinase, binds the ran Gtpase and regulates mitotic progression. Genes Dev 16:1640–1658
Roig J, Groen A, Caldwell J, Avruch J (2005) Active Nercc1 protein kinase concentrates at centrosomes early in mitosis and is necessary for proper spindle assembly. Mol Biol Cell 16:4827–4840
Rosenblatt J, Cramer LP, Baum B, Mcgee KM (2004) Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell 117:361–372
Salem H, Rachmin I, Yissachar N, Cohen S, Amiel A, Haffner R, Lavi L, Motro B (2010) Nek7 kinase targeting leads to early mortality, cytokinesis disturbance and polyploidy. Oncogene 29:4046–4057
Silkworth WT, Nardi IK, Paul R, Mogilner A, Cimini D (2012) Timing of centrosome separation is important for accurate chromosome segregation. Mol Biol Cell 23:401–411
Smith E, Hegarat N, Vesely C, Roseboom I, Larch C, Streicher H, Straatman K, Flynn H, Skehel M, Hirota T, Kuriyama R, Hochegger H (2011) Differential control of Eg5-dependent centrosome separation by Plk1 and Cdk1. EMBO J 30:2233–2245
So CH, Michal A, Komolov KE, Luo J, Benovic JL (2013) G protein-coupled receptor kinase 2 (Grk2) is localized to centrosomes and mediates epidermal growth factor-promoted centrosomal separation. Mol Biol Cell 24:2795–2806
Splinter D, Tanenbaum ME, Lindqvist A, Jaarsma D, Flotho A, Yu KL, Grigoriev I, Engelsma D, Haasdijk ED, Keijzer N, Demmers J, Fornerod M, Melchior F, Hoogenraad CC, Medema RH, Akhmanova A (2010) Bicaudal D2, dynein, and kinesin-1 associate with nuclear pore complexes and regulate centrosome and nuclear positioning during mitotic entry. Plos Biol 8, E1000350
Suijkerbuijk SJ, Vleugel M, Teixeira A, Kops GJ (2012) Integration of kinase and phosphatase activities by Bubr1 ensures formation of stable kinetochore-microtubule attachments. Dev Cell 23:745–755
Tanenbaum ME, Medema RH (2010) Mechanisms of centrosome separation and bipolar spindle assembly. Dev Cell 19:797–806
Tanenbaum ME, Macurek L, Galjart N, Medema RH (2008) Dynein, Lis1 and Clip-170 counteract Eg5-dependent centrosome separation during bipolar spindle assembly. EMBO J 27:3235–3245
Tanenbaum ME, Akhmanova A, Medema RH (2010) Dynein at the nuclear envelope. EMBO Rep 11:649
Toso A, Winter JR, Garrod AJ, Amaro AC, Meraldi P, Mcainsh AD (2009) Kinetochore-generated pushing forces separate centrosomes during bipolar spindle assembly. J Cell Biol 184:365–372
Van Heesbeen RG, Raaijmakers JA, Tanenbaum ME, Medema RH (2013) Nuclear envelope-associated dynein cooperates with Eg5 to drive prophase centrosome separation. Commun Integr Biol 6, E23841
Wang G, Jiang Q, Zhang C (2014) The role of mitotic kinases in coupling the centrosome cycle with the assembly of the mitotic spindle. J Cell Sci 127:4111–4122
Yissachar N, Salem H, Tennenbaum T, Motro B (2006) Nek7 kinase is enriched at the centrosome, and is required for proper spindle assembly and mitotic progression. FEBS Lett 580:6489–6495
Zhang Y, Foreman O, Wigle DA, Kosari F, Vasmatzis G, Salisbury JL, Van Deursen J, Galardy PJ (2012) Usp44 regulates centrosome positioning to prevent aneuploidy and suppress tumorigenesis. J Clin Invest 122:4362–4374
Zhao J, Zou Y, Liu H, Wang H, Zhang H, Hou W, Li X, Jia X, Zhang J, Hou L, Zhang B (2014) TEIF associated centrosome activity is regulated by Egf/Pi3k/Akt signaling. Biochim Biophys Acta 1843:1851–1864
Acknowledgments
This work was supported by grants from the National Institutes of Health (CA126828 and CA168709 to J.M.v.D.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Daniela Cimini and Giulia Guarguaglini
Janine H. van Ree and Hyun-Ja Nam shared first authors with equal contributions.
Rights and permissions
About this article
Cite this article
van Ree, J.H., Nam, HJ. & van Deursen, J.M. Mitotic kinase cascades orchestrating timely disjunction and movement of centrosomes maintain chromosomal stability and prevent cancer. Chromosome Res 24, 67–76 (2016). https://doi.org/10.1007/s10577-015-9501-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-015-9501-9