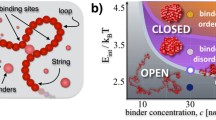
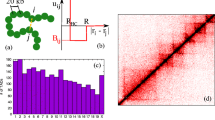
Abstract
We review the picture of chromatin large-scale 3D organization emerging from the analysis of Hi-C data and polymer modeling. In higher mammals, Hi-C contact maps reveal a complex higher-order organization, extending from the sub-Mb to chromosomal scales, hierarchically folded in a structure of domains-within-domains (metaTADs). The domain folding hierarchy is partially conserved throughout differentiation, and deeply correlated to epigenomic features. Rearrangements in the metaTAD topology relate to gene expression modifications: in particular, in neuronal differentiation models, topologically associated domains (TADs) tend to have coherent expression changes within architecturally conserved metaTAD niches. To identify the nature of architectural domains and their molecular determinants within a principled approach, we discuss models based on polymer physics. We show that basic concepts of interacting polymer physics explain chromatin spatial organization across chromosomal scales and cell types. The 3D structure of genomic loci can be derived with high accuracy and its molecular determinants identified by crossing information with epigenomic databases. In particular, we illustrate the case of the Sox9 locus, linked to human congenital disorders. The model in-silico predictions on the effects of genomic rearrangements are confirmed by available 5C data. That can help establishing new diagnostic tools for diseases linked to chromatin mis-folding, such as congenital disorders and cancer.







Similar content being viewed by others
Abbreviations
- CTCF:
-
CCCTC-binding factor
- FISH:
-
Fluorescence in situ hybridization
- mESC:
-
mouse embryonic stem cell
- NPC:
-
Neural precursor cell
- SBS:
-
Strings & Binders
- TAD:
-
Topologically associated domain
References
Annunziatella C, Chiariello AM, Bianco S, Nicodemi M (2016) Polymer models of the hierarchical folding of the Hox-B chromosomal locus. Phys Rev E 94:042402
Barbieri M, Chotalia M, Fraser J, Lavitas LM, Dostie J, Pombo A, Nicodemi M (2012) Complexity of chromatin folding is captured by the strings and binders switch model. Proc Natl Acad Sci U S A 109:16173–16178
Bickmore WA, van Steensel B (2013) Genome architecture: domain organization of interphase chromosomes. Cell 152:1270–1284
Bohn M, Heermann DW (2010) Diffusion-driven looping provides a consistent framework for chromatin organisation. PLoS One 5:e12218
Boulos RE, Drillon G, Argoul F, Arneodo A, Audit B (2015) Structural organization of human replication timing domains. FEBS Lett 589:2944–2957
Brackley CA, Brown JM, Waithe D et al (2016b) Predicting the three-dimensional folding of cis-regulatory regions in mammalian genomes using bioinformatic data and polymer models. Genome Biol 17:59
Brackley CA, Taylor S, Papantonis A, Cook PR, Marenduzzo D (2013) Nonspecific bridging-induced attraction drives clustering of DNA-binding proteins and and genome organisation. Proc Natl Acad Sci U S A 110:E3605–E3611
Brackley CA, Johnson J, Kelly S, Cook PR, Marenduzzo D (2016a) Simulated binding of transcription factors to active and inactive regions folds human chromosomes into loops, rosettes and topological domains. Nucl Acids Res 44:3503–3512
Cheng TMK, Heeger S, Chaleil RAG et al (2015) A simple biophysical model emulates budding yeast chromosome condensation. eLife 4:e05565
Chiariello AM, Annunziatella C, Bianco S, Esposito A, Nicodemi M (2016) Polymer physics of chromosome large-scale 3d organisation. Scientific Reports 6:29775
Dekker J, Mirny L (2016) 3D genome as moderator of chromosomal communication. Cell 164:1110–1121
Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B (2012) Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485:376–380
Di Stefano M, Paulsen J, Lien TG, Hovig E, Micheletti C (2016) Hi-C-constrained physical models of human chromosomes recover functionally-related properties of genome organization. Scientific Reports 6:35985
Franke M, Ibrahim DM et al (2016) Formation of new chromatin domains determines pathogenicity of genomic duplications. Nature 538:265–269
Fraser J, Ferrai C, Chiariello AM et al (2015) Hierarchical folding and reorganisation of chromosomes are linked to transcriptional changes during cellular differentiation. Mol Syst Biol 11:852
Fudenberg G, Imakaev M, Lu C et al (2016) Formation of chromosomal domains by loop extrusion. Cell Rep 15:1–12
Giorgietti L, Galupa R, Nora EP et al (2014) Predictive polymer modeling reveals coupled fluctuations in chromosome conformation and transcription. Cell 157:950–963
Jost D, Carrivain P, Cavalli G, Vaillant C (2014) Modeling epigenome folding: formation and dynamics of topologically associated chromatin domains. Nucleic Acids Res 42:9553–9561
Kreth G, Finsterle J, von Hase J, Cremer M, Cremer C (2004) Radial arrangement of chromosome territories in human cell nuclei: a computer model approach based on gene density indicates a probabilistic global positioning code. Biophys J 86:2803–2812
Lanctôt C, Cheutin T, Cremer M, Cavalli G, Cremer T (2007) Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat Rev Genet 8:104–115
Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, Sandstrom R, Bernstein B, Bender MA, Groudine M, Gnirke A, Stamatoyannopoulos J, Mirny LA, Lander ES, Dekker J (2009) Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326:289–293
Lupiáñez DG, Kraft K, Heinrich V et al (2015) Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell 161:1
Marenduzzo D, Micheletti C, Cook PR (2006) Entropy-driven genome organisation. Biophys J 90:3712–3721
Misteli T (2007) Beyond the sequence: cellular organization of genome function. Cell 128:787–800
Nicodemi M, Pombo A (2014) Models of chromosome structure. Curr Opin Cell Biol 28C:90–95
Nicodemi M, Prisco A (2009) Thermodynamic pathways to genome spatial organization in the cell nucleus. Biophys J 96:2168–2177
Nora EP, Lajoie BR, Schulz EG, Giorgetti L, Okamoto I, Servant N, Piolot T, van Berkum NL, Meisig J, Sedat J, Gribnau J, Barillot E, Bluthgen N, Dekker J, Heard E (2012) Spatial partitioning of the regulatory landscape of the X-inactivation Centre. Nature 485:381–385
Ong CT, Corces VG (2014) CTCF: an architectural protein bridging genome topology and function. Nat Rev Genet 15:234–246
Phillips-Cremins JE, Sauria ME, Sanyal A, Gerasimova TI, Lajoie BR, Bell JS, Ong CT, Hookway TA, Guo C, Sun Y, Bland MJ, Wagstaff W, Dalton S, McDevitt TC, Sen R, Dekker J, Taylor J, Corces VG (2013) Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell 153:1281–1295
Rosa A, Everaers R (2008) Structure and dynamics of interphase chromosomes. PLoS Comput Biol 4:e1000153
Sanborn AL, Rao SSP, Huang S-C et al (2015) Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc Natl Acad Sci U S A 112:E6456–E6465
Scialdone A, Cataudella I, Barbieri M, Prisco A, Nicodemi M (2011) Conformation regulation of the X chromosome inactivation center: a model. PLoS Comput Biol 7:e1002229
Schmitt AD, Hu M, Jung I, Xu Z, Qiu Y, Tan CL, Li Y, Lin S, Lin Y, Barr CL, Ren B (2016) A compendium of chromatin contact maps reveals spatially active regions in the human genome. Cell Rep 17:2042–2059
Tanay A, Cavalli G (2013) Chromosomal domains: epigenetic contexts and functional implications of genomic compartmentalization. Current Opinion in Genetics & Development 23:197–203
The ENCODE Project Consortium (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489:57–74
Valton AL, Dekker J (2016) TAD disruption as oncogenic driver. Curr Opin Genet Dev 36:34–40
Acknowledgements
Work supported by grants to MN from the NIH ID 1U54DK107977-01 and CINECA ISCRA ID HP10CYFPS5 and HP10CRTY8P. MN also acknowledges computer resources from INFN, CINECA, and Scope at the University of Naples.
Author Contributions
M.N. designed the study; S.B., A.M.C., C.A., M.N. developed the project; A.M.C., C.A., S.B., A.E. run the computer simulations and performed the analyses; A.M.C., C.A., S.B., M.N. wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible editor: Nick Gilbert and Davide Marenduzzo.
Simona Bianco, Andrea M. Chiariello, and Carlo Annunziatella have equal contribution.
Rights and permissions
About this article
Cite this article
Bianco, S., Chiariello, A.M., Annunziatella, C. et al. Predicting chromatin architecture from models of polymer physics. Chromosome Res 25, 25–34 (2017). https://doi.org/10.1007/s10577-016-9545-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-016-9545-5