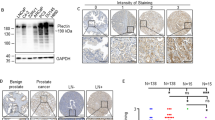
Abstract
Secreted protein, acidic and rich in cysteine (SPARC, also known as osteonectin or BM-40) is a glycoprotein component of the extracellular matrix that has been reported to be involved with a variety of cellular processes. Although SPARC expression levels are frequently altered in a variety of tumor types, the exact implications of deregulated SPARC expression—whether it promotes, inhibits or has no effect on tumor progression—have remained unclear. Our recent gene expression analyses have shown that SPARC is significantly downregulated in highly metastatic human prostate cancer cells. To test the role of endogenous SPARC in tumorigenesis directly, we examined cancer progression and metastasis in SPARC+/− and SPARC−/− mice using two separate transgenic mouse tumor models: transgenic adenocarcinoma of the mouse prostate (TRAMP) and murine mammary tumor virus-polyoma middle T (MMTV-PyMT). Surprisingly, in both instances, we found that loss of SPARC had no significant effects on tumor initiation, progression or metastasis. Tumor angiogenesis and collagen deposition were also largely unaffected. Our results indicate that, although differential SPARC expression may be a useful marker of aggressive, metastasis-prone tumors, loss of SPARC is not sufficient either to promote or to inhibit cancer progression in two spontaneous mouse tumor models.





Similar content being viewed by others
Abbreviations
- SPARC:
-
Secreted protein, acidic and rich in cysteine
- TRAMP:
-
Transgenic adenocarcinoma of the mouse prostate
- MMTV-PyMT:
-
Murine mammary tumor virus-polyoma middle T
- DL:
-
Dorsolateral prostate
- VN:
-
Ventral prostate
- SOI:
-
Surgical orthotopic implantation
References
Brekken RA, Sage EH (2001) SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol 19:816–827
Framson PE, Sage EH (2004) SPARC and tumor growth: where the seed meets the soil? J Cell Biochem 92:679–690
Bradshaw AD, Sage EH (2001) SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 107:1049–1054
Bradshaw AD, Reed MJ, Sage EH (2002) SPARC-null mice exhibit accelerated cutaneous wound closure. J Histochem Cytochem 50:1–10
Murphy-Ullrich JE (2001) The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest 107:785–790
Sage H, Vernon RB, Funk SE, Everitt EA, Angello J (1989) SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J Cell Biol 109:341–356
Brekken RA et al (2003) Enhanced growth of tumors in SPARC null mice is associated with changes in the ECM. J Clin Invest 111:487–495
Motamed K, Sage EH (1998) SPARC inhibits endothelial cell adhesion but not proliferation through a tyrosine phosphorylation-dependent pathway. J Cell Biochem 70:543–552
Kupprion C, Motamed K, Sage EH (1998) SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells (1998). J Biol Cell 273:29635–29640
Schiemann BJ, Neil JR, Schiemann WP (2003) SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-beta-signaling system. Mol Biol Cell 14:3977–3988
Tremble PM, Lane TF, Sage EH, Werb Z (1993) SPARC, a secreted protein associated with morphogenesis and tissue remodeling, induces expression of metalloproteinases in fibroblasts through a novel extracellular matrix-dependent pathway. J Cell Biol 121:1433–1444
Shankavaram UT, DeWitt DL, Funk SE, Sage EH, Wahl LM (1997) Regulation of human monocyte matrix metalloproteinases by SPARC. J Cell Physiol 173:327–334
Wang CS, Lin KH, Chen SL, Chan YF, Hsueh S (2004) Overexpression of SPARC gene in human gastric carcinoma and its clinic-pathologic significance. Br J Cancer 91:1924–1930
Chin D et al (2005) Novel markers for poor prognosis in head and neck cancer. Int J Cancer 113:789–797
Yamanaka M et al (2001) Analysis of the gene expression of SPARC and its prognostic value for bladder cancer. J Urol 166:2495–2499
Thomas R, True LD, Bassuk JA, Lange PH, Vessella RL (2000) Differential expression of osteonectin/SPARC during human prostate cancer progression. Clin Cancer Res 6:1140–1149
Bail BL et al (1999) Osteonectin/SPARC is overexpressed in human hepatocellular carcinoma. J Pathol 189:46–52
De S et al (2003) Molecular pathway for cancer metastasis to bone. J Biol Chem 278:39044–39050
Ledda MF et al (1997) Suppression of SPARC expression by antisense RNA abrogates the tumorigenicity of human melanoma cells. Nat Med 3:171–176
Robert G et al (2006) SPARC represses E-cadherin and induces mesenchymal transition during melanoma development. Cancer Res 66:7516–7523
Jacob K, Webber M, Benayahu D, Kleinman HK (1999) Osteonectin promotes prostate cancer cell migration and invasion: a possible mechanism for metastasis to bone. Cancer Res 59:4453–4457
Yiu GK et al (2001) SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol 159:609–622
Koblinski JE et al (2005) Endogenous osteonectin/SPARC/BM-40 expression inhibits MDA-MB-231 breast cancer cell metastasis. Cancer Res 65:7370–7377
Tai IT, Dai M, Owen DA, Chen LB (2005) Genome-wide expression analysis of therapy-resistant tumors reveals SPARC as a novel target for cancer therapy. J Clin Invest 115:1492–1502
Chlenski A et al (2006) SPARC expression is associated with impaired tumor growth, inhibited angiogenesis and changes in the extracellular matrix. Int J Cancer 118:310–316
Puolakkainen PA, Brekken RA, Muneer S, Sage EH (2004) Enhanced growth of pancreatic tumors in SPARC-null mice is associated with decreased deposition of extracellular matrix and reduced tumor cell apoptosis. Mol Cancer Res 2:215–224
Sangaletti S, Stoppacciaro A, Guiducci C, Torrisi MR, Colombo MP (2003) Leukocytes, rather than tumor-produced SPARC, determines stroma and collagen type IV deposition in mammary carcinoma. J Exp Med 198:1475–1485
Iruela-Arispe ML (1995) Expression of SPARC during development of the chicken chorioallantoic membrane: evidence for regulated proteolysis in vivo. Mol Biol Cell 6:327–343
Aycock RL, Bradshaw AC, Sage EH, Starcher B (2004) Development of UV-induced squamous cell carcinomas is suppressed in the absence of SPARC. J Invest Dermatol 123:592–599
Sansom OJ, Mansergh F, Evans M, Wilkins J, Clarke A (2007) Deficiency of SPARC suppresses intestinal tumourigenesis in APCMin/+ mice. Gut 56:1410–1414
Wong SY et al (2007) Protein 4.1B suppresses prostate cancer progression and metastasis. Proc Natl Acad Sci USA 104:12784–12789
Chang XH et al (1999) Improved metastatic animal model of human prostate carcinoma using surgical orthotopic implantation (SOI). Anticancer Res 19:4199–4202
An Z, Wang X, Geller J, Moossa AR, Hoffman RM (1998) Surgical orthotopic implantation allows high lung and lymph node metastatic expression of human prostate carcinoma cell line PC-3 in nude mice. Prostate 34:169–174
Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW (1979) Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol 17:16–23
Norose K et al (1998) SPARC deficiency leads to early-onset cataractogenesis. Invest Ophthalmol Vis Sci 39:2674–2680
Greenberg NM et al (1995) Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA 92:3439–3443
Guy CT, Cardiff RD, Muller WJ (1992) Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 12:954–961
Hurwitz AA, Foster BA, Allison JP, Greenberg NM, Kwon ED (2001) The TRAMP mouse as a model for prostate cancer. In: Current protocols in immunology. Wiley, New York
Armed Forces Institute of Pathology Staff, Prophet EB (1992) Afip laboratory methods in histotechnology. American Registry of Pathology, Washington
Li C, Wong WH (2001) Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol 2:0032.1–0032.11
Rhodes DR et al (2004) ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 6:1–6
van’t Veer LJ et al (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–535
Wong SY et al (2005) Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res 65:9789–9798
Dhanasekaran SM et al (2001) Delineation of prognostic biomarkers in prostate cancer. Nature 412:822–826
Lapointe J et al (2004) Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA 101:811–816
Welsh JB et al (2001) Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res 61:5974–5978
Smid M, Dorssers LC, Jenster G (2003) Venn Mapping: clustering of heterologous microarray data based on the number of co-occurring differentially expressed genes. Bioinformatics 19:2065–2071
Chlenski A et al (2007) SPARC enhances tumor stroma formation and prevents fibroblast activation. Oncogene 26:4513–4522
Bhowmick NA, Neilson EG, Moses HL (2004) Stromal fibroblasts in cancer initiation and progression. Nature 432:332–337
Kang Y et al (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 6:537–549
Acknowledgements
We are very grateful to Dr. E. Helene Sage and Sarah Funk (Benaroya Research Institute, Seattle, WA) for SPARC-deficient mice; Dr. Lei Xu (MIT) for MMTV-PyMT mice; Dr. Ailin Bai (MIT) for TRAMP mice; Jane Trevithick for technical assistance; and the MIT Division of Comparative Medicine for animal maintenance. This work was supported by grants from the NIH (RO1CA17007); from the Virginia and D. K. Ludwig Fund for Cancer Research; from the Prostate Cancer Foundation; from the National Cancer Institute’s Integrative Cancer Biology Program (U54-CA112967); and from the Howard Hughes Medical Institute, of which R. O. H. is an Investigator. S. Y. W. was further supported by an NIGMS Predoctoral Training Grant to the MIT Biology Department and by a David H. Koch Research Fellowship from the Center for Cancer Research.
Financial support
NIH (RO1CA17007); Virginia and D. K. Ludwig Fund for Cancer Research; Prostate Cancer Foundation; Integrative Cancer Biology Program (National Cancer Institute, U54-CA112967); Howard Hughes Medical Institute. S. Y. W. was further supported by an NIGMS Predoctoral Training Grant and by a David H. Koch Research Fellowship from the Center for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, S.Y., Crowley, D., Bronson, R.T. et al. Analyses of the role of endogenous SPARC in mouse models of prostate and breast cancer. Clin Exp Metastasis 25, 109–118 (2008). https://doi.org/10.1007/s10585-007-9126-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-007-9126-2