Abstract
The worldwide pandemic of COVID-19, caused by the virus SARS-CoV-2, continues to cause significant morbidity and mortality in both low- and high-income countries. Although COVID-19 is predominantly a respiratory illness, other systems including gastrointestinal (GI) system and liver may be involved because of the ubiquitous nature of ACE-2 receptors in various cell lines that SARS-CoV-2 utilizes to enter host cells. It appears that GI symptoms and liver enzyme abnormalities are common in COVID-19. The involvement of the GI tract and liver correlates with the severity of disease. A minority (10–20%) of patients with COVID-19 may also present initially with only GI complaints. The most common GI symptoms are anorexia, loss of smell, nausea, vomiting, and diarrhea. Viral RNA can be detected in stool in up to 50% of patients, sometimes even after pharyngeal clearance, but it is unclear whether fecal–oral transmission occurs. Liver enzymes are elevated, usually mild (2–3 times), in a substantial proportion of patients. There are many confounding factors that could cause liver enzyme abnormalities including medications, sepsis, and hypoxia. Although infection rates in those with preexisting liver disease are similar to that of general population, once infected, patients with liver disease are more likely to have a more severe disease and a higher mortality. There is a paucity of objective data on the optimal preventive or management strategies, but few recommendations for GI physicians based on circumstantial evidence are discussed.
Similar content being viewed by others
Introduction
The ongoing pandemic of coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) continues to be a major concern in many countries around the world [1]. Of those who develop COVID-19, ~ 15% develop severe disease and ~ 5% become critically ill resulting in death in half of them. To date, the highest case fatality rates from COVID-19 have been reported in the USA, Brazil, the UK, Italy, Spain, France, and Mexico. This novel coronavirus infection, which may have originated from the wet markets of Wuhan Province in China in December 2019, causes predominantly acute respiratory symptoms. As of July 8, 2020, more than 12 million people have been diagnosed with this infection worldwide and of these, over 550,000 have died [2]. The USA has the highest number of fatal cases in the world with more than 135,000 deaths. The disease has overwhelmed health systems all over the world including high-income countries such as the USA, UK, Italy, Spain, and France and is rapidly spreading in Russia, India, and Brazil.
The incubation period for this infection is 0–14 days, and it has been speculated that up to 20–60% of infected people could be asymptomatic [3,4,5]. Data from recently available antibody testing indicate that the seroprevalence of COVID-19 ranges anywhere between 4 and 10% depending on the prevalence of infection in a particular region, implying that a vast majority of the population has not been exposed to the virus yet [6, 7]. Transmission of the virus occurs via respiratory droplets or by direct contact with contaminated surfaces, but aerosol transmission may occur as well [8]. SARS-CoV-2 binds to Angiotensin Converting Enzyme-2 (ACE-2) receptors on host target cells to gain entry and replicate. The receptor binding domain of the virus is very similar to that of the virus that caused the SARS–CoV-1 epidemic of 2002–2003 [9]. ACE-2 receptors are ubiquitous and are expressed in many human cell lines including the intestinal epithelium and hepatobiliary system. In synergy with ACE-2 receptor, another host cell protein, transmembrane serine protease 2 (TMPRSS2) also plays a key role in facilitating viral entry into the host by cleaving and activating the spike (S) glycoprotein present on the envelope of SARS-CoV-2 [10, 11]. TMPRSS2 is abundantly expressed in the ileum and colon and along with ACE-2 receptors could perhaps explain various gastrointestinal (GI) and hepatobiliary manifestations of COVID-19 (Fig. 1).
Gastrointestinal Manifestations
Fever, cough, and fatigue are the most common symptoms of COVID-19. More than 80% of patients also report gustatory and olfactory disturbance with loss of smell and taste sensation [12]. However, there is a wide variation in the reported incidence of GI symptoms in COVID-19 patients (Table 1) [13, 14]. Loss of appetite is the most common symptom, followed by nausea, vomiting, and diarrhea. Nausea and vomiting in these patients are highly variable ranging anywhere between 5 and 66% [13, 14]. Diarrhea as a presenting complaint is seen in 3–37% of patients [12,13,14,15]. Few patients may present only with diarrhea without any significant respiratory complaints, and this could lead to a delay in diagnosis [16, 17]. In one study, it was shown that the time interval between symptom onset and hospital admission was longer in COVID-19 patients who had exclusively digestive symptoms compared to patients with only respiratory complaints (16.0 ± 7.7 vs. 11.6 ± 5.1 days, p < 0.001) [18]. Moreover, patients with only digestive complaints had a longer time duration between onset of symptoms and viral clearance compared to their counterparts. (40.9 vs. 33.5 days, p < 0.001). The presence of GI symptoms appears to correlate with more severe disease, and moreover, diarrhea and abdominal pain may get worse as the disease progresses [15,16,17,18,19].
The largest study evaluating GI symptoms and signs was reported from the viral epicenter in Wuhan, China [13]. In this retrospective study of 1141 hospitalized patients in a single hospital over a period of 7 weeks, 16% of patients presented with gastrointestinal symptoms alone. Of these 183 patients, the most common symptom was loss of appetite. Nausea and vomiting occurred in two-thirds of patients, diarrhea in about one-third, and abdominal pain in one-quarter of those who presented with GI symptoms. Liver enzymes were mildly elevated in the majority of patients. In another study of 204 patients from Hubei province in China, 99 patients (48.5%) had gastrointestinal symptoms including anorexia in 83 (83.8%), diarrhea in 29 (29.3%), vomiting in 8 (8.1%), and abdominal pain in 4 (4.0%), with some patients having multiple symptoms [16]. As in the previous study, a minority (n = 7) had only GI symptoms; authors noted that there was a delay in hospitalization in these patients from the onset of symptoms perhaps because diarrhea is considered non-specific for COVID-19. In a separate study of 651 patients from China, 74 (11.4%) presented with at least one GI symptom including nausea, vomiting, or diarrhea [19]. In this cohort of patients, 17 (23%) had severe or critical illness compared to 47 (8.1%) without GI symptoms. Authors also found family clustering in 23 (31.1%) patients with GI symptoms compared to 118 (20.5%) without GI symptoms. Patients with GI symptoms had significantly higher rates of fever > 38.5 °C in 29 (39%), fatigue 23 (31.1%), shortness of breath 8 (10.8%) and headache, and 16 (21.62%). Other studies from China, however, have reported lower incidence of GI symptoms. In one study of 1099 patients, only 5% (55/1099) presented with nausea or vomiting and 3.8% (42/1099) with diarrhea [14]. Another study of 138 hospitalized patients reported that only 10.1% (14/138) of patients had diarrhea and/or nausea [19]. A meta-analysis published recently summarized that 17.6% develop gastrointestinal symptoms and it is worse among those with severe disease (17.1 vs. 11.8%) when compared to those with non-severe disease [18]. Most of these studies that were included in this meta-analysis were from China and included a heterogeneous inpatient population and hence cannot be generalized.
Data from the USA are consistent with Chinese studies and report a high prevalence (23–35%) of gastrointestinal symptoms in patients infected with COVID-19 [21,22,23,24]. According to one observational study, COVID-19 patients who had concomitant diarrhea had a sevenfold higher likelihood for hospitalization (OR 4.84 95% CI 1.68–13.94), while those with nausea and vomiting had 4 times higher risk (OR = 7.58, 95% CI 2.49–20.02) [21]. Another prospective case control study of 340 patients (SARS-CoV-2 positive = 101, SARS-CoV-2 negative = 239) found that the specificity for COVID-19 infection was 99% if patients presented with diarrhea and anorexia in addition to fever, loss of taste, and smell [22].
There are also reports of colitis and colonic ileus in hospitalized patients who were positive for SARS-CoV-2. A case series from the USA illustrated three patients who presented with diffuse pain abdomen, of which two had inflammation of the large bowel, while one had colonic ileus and air in the bowel wall [25]. An isolated report from China describes the case of an elderly male who presented to the hospital with multiple episodes of frank hematochezia with no respiratory complaints [26]. CT scan and MRI of the abdomen were unremarkable and colonoscopy failed to reveal any hemorrhagic sites. On day 7, he developed lung infiltrates and tested positive for SARS-CoV-2. The authors concluded that hematochezia was most probably secondary to the virus since no other pathology could be identified. Whether colonic involvement occurs as a result of direct cellular infection from coronavirus, or whether it is a result of profound inflammatory response secondary to COVID-19 remains to be elucidated.
There is concern among gastroenterologists whether patients with IBD are at increased risk of infection secondary to their state of immunosuppression. A study in Italy analyzed 79 patients with IBD (Crohn’s disease = 32, and ulcerative colitis = 47) and COVID-19 and found that active IBD, older age, and comorbidities were associated with increased risk of developing pneumonia, respiratory support, hospitalization, and death [27]. IBD treatments with corticosteroids or other immunosuppressive drugs were not associated with a negative outcome. The authors also claimed that patients with IBD may not be at a higher risk of infection with coronavirus compared to the general population since they were able find only 79 patients who had both IBD and COVID-19, which is relatively a smaller number given the higher incidence of infection in Northern Italy at the height of the pandemic.
We will have a better understanding of the gastrointestinal manifestations of COVID-19 as more data are published from Europe and North America, especially in both the outpatient and inpatient populations.
Fecal–Oral Transmission
Another pertinent question is whether SARS-CoV-2 is transmissible by the fecal–oral route [28, 29]. In a small study published from Hong Kong, of 59 patients with confirmed COVID-19 infections, 15 patients had gastrointestinal symptoms, and 9 patients had stool RNA positivity [30]. Stool viral RNA was detected in 38.5 and 8.7% among those with and without diarrhea, respectively (p = 0.02). The median fecal viral load was 5.1 log10cpm in patients with diarrhea versus 3.9 log10cpm in patients without diarrhea (p = 0.06). This study is too small to make any firm conclusions and needs further confirmation. Apart from this very small study, there are no other studies that have examined GI symptoms and active viral RNA replication virus in the intestinal tract.
There are studies that have reported the presence of viral RNA in the stool even after nasopharyngeal swabs had tested negative raising the possibility of fecal–oral transmission especially in communities with poor hygiene [30, 31]. In one of these case reports, 10 days after initial presentation with respiratory symptoms and fever, multiple pharyngeal samples over a period of 7 days were negative for SARS-CoV-2, but a separate fecal sample was positive [32]. In a study from Singapore, 50% of patients had detectable RNA in their stool samples, but detection of RNA in the stool did not correlate with GI symptoms [33]. A recent meta-analysis of 60 studies consisting of 4243 patients showed that 48% patients with the infection may have detectable RNA in the stool and interestingly they found that a majority (70%) of patients tested positive after their respiratory specimens had cleared the virus [18].
The time taken for viral RNA clearance after recovery was examined in a recent study from China by analyzing samples of oropharyngeal swab, stool, urine, and serum with RT-PCR [31]. In this study, the median duration from the onset of symptoms to first negative RNA test from oropharyngeal swab was 9.5 (6.0–11.0) days, while 16.7% (11/67) had positive stool RNA for a median of 11.0 (9.0–16.0) days. Investigators also reported that the duration of viral RNA in the stool was longer (20 days vs. 11 days, p < 0.0001) in those were treated with glucocorticoids compared to those who did not receive steroids. This study along with the small study that showed a higher prevalence of stool RNA positivity in those with diarrhea may suggest that there is a potential for fecal–oral transmission of this virus [18, 34]. The high viral load in those with diarrhea suggests GI involvement. It is unlikely that positive RNA in the stool detected by RT-PCR is due to noninfectious viral particles or small amounts of viruses that originated in the respiratory tract. These preliminary observations merit further examination since there is a potential, although unproven, for fecal–oral transmission risk for many days even after clinical recovery.
Pancreas Involvement
ACE2 receptors expressed on pancreatic islet cells are a potential focus of entry for SARS-CoV-2 and could theoretically cause acute pancreatitis (AP). There are isolated case reports from Europe and the USA confirming the same. A case series from Denmark reported 2 cases of severe AP in COVID 19 patients admitted to the ICU, and these patients had no other risk factors including alcohol, gall stones, hypotension, drugs, trauma, hypertriglyceridemia, or hypercalcemia [35]. Both patients had elevated levels of serum lipase in addition to abdominal pain and had a Modified Glasgow Acute Pancreatitis Score of 5. Another report from the USA described a case of acute pancreatitis and ARDS in a patient who tested positive for SARS-CoV-2 [36]. To date, the largest report on the impact of SARS-CoV-2 on pancreas was reported from China where nine out of 52 (18%) patients with COVID pneumonia were found have elevated serum amylase and lipase [37]. They also found that patients with pancreatic injury had a higher incidence of serious illness, anorexia, diarrhea, as well as higher levels of AST, creatinine, LDH, and ESR. It appears that pancreatic involvement is extremely rare in COVID-19.
Gall Bladder Involvement
Gallbladder involvement although rare with SARS-CoV-2 has also been described in the literature [38, 39]. One report describes three hospitalized patients who developed acute pain abdomen while recovering from COVID-19 pneumonia. CT scan of the abdomen was consistent with acute acalculous cholecystitis, and three all underwent emergent laparoscopic cholecystectomy which confirmed gangrenous cholecystitis [38]. Micro-thrombosis which is a well-established cause of tissue hypoxia in patients with COVID-19 may be responsible for gall bladder ischemia resulting in acalculous cholecystitis.
Liver Manifestations
Abnormal Liver Enzymes
Elevation of transaminases was reported in 22% of patients in a large series, but other smaller case series have reported higher incidence ranging from 30 to 50% (Table 2) [14, 15, 40]. It appears that the elevated liver enzymes are more often seen in severe disease and this could be a reflection of confounding factors such as medications, preexisting liver disease (hepatitis B was reported in up to 10% of patients from China), sepsis, and hypoxia/reperfusion injury. A meta-analysis of early reports from China showed a significant association between severe/critical COVID-19 and elevations in AST (pooled mean difference 11.7 IU/L, 95% CI 3.0, 20.4, p = 0.009) and total bilirubin (pooled mean difference 0.14 mg/dL, 95% CI 0.06, 0.22, p = 0.0005) [41]. Patients who presented with abnormal liver enzymes at admission were found to have an increased risk of progression to severe pneumonia in one study [42]. ACE2 receptors are highly expressed in cholangiocytes, but cholestatic liver disease has been reported very rarely and most patients with elevated AST/ALT have normal alkaline phosphatase.
Concomitant medications or preexisting liver diseases are most likely the cause of mild liver test abnormalities in COVID-19. Remdesivir, an antiviral drug that was recently approved for emergency use by the FDA after promising preliminary trials in COVID-19, is associated with increased LFTs. In the largest trial (n = 1073) that evaluated the efficacy and safety of remdesivir, 4.1% experienced elevated aminotransferase levels [43]. Another study found that a rise in alanine aminotransferase (ALT) levels was higher in patients who received a 10-day course of remdesivir when compared to a 5-day course (6 vs. 8%), and as a result of elevated liver enzymes 3.6% in the 10-day group and 2.5% in the 5-day group discontinued treatment. Elevation of ALT > 5 times the upper limit of normal (ULN) at baseline is considered an exclusion criterion for remdesivir trials [44]. Similarly, reports from China indicate lopinavir/ritonavir, another antiviral combination is associated with an increased risk of hepatic injury, although this antiviral is combination is found to be ineffective against COVID-19 [45].
The prevalence of chronic liver disease (CLD) among Chinese patients with COVID-19 was relatively small (2–11%) [46]. Similar numbers (3%) were also reported in a large study (n = 1591) from Italy and in their study, 75% of patients with underlying CLD was > 50 years of age [47]. Moreover, two meta-analyses showed that the prevalence of CLD was only 3% in COVID-19 patients and a majority of them had either underlying chronic hepatitis B virus or hepatitis C virus infection [48, 49].
Liver Failure
Although there are isolated case reports of very high transaminases, acute liver failure has not been reported with COVID-19 infection [50]. It is more than likely that very high liver enzymes reported in isolated case reports are related to hypoxia–reperfusion injury, uncontrolled immune reaction, or medications and less likely due to cytopathic effect of the virus based on current evidence. A case report from the USA described the first case of acute on chronic liver failure (ACLF) due to SARS-CoV-2 [51]. The patient had cirrhosis secondary to alcohol and presented with acute liver failure (total bilirubin of 14.5 mg/dL and INR of 2.6) and pneumonia. Her workup was positive for COVID-19, and liver failure was deemed secondary to the same. She had a successful outcome after treatment and was discharged home after 7 days of hospitalization. In a small study from Asia, APASL ACLF Research Consortium (AARC) reported ACLF (using APASL criteria) in five of 43 (11.6%) patients with known cirrhosis (25 with compensated and 18 with decompensated cirrhosis) [52]. In this study, two of 25 (8%) with compensated cirrhosis and six of 18 (33.3%) with decompensated cirrhosis died compared to only 2.1% of patients CLD without cirrhosis. It is difficult to make any firm conclusions from this retrospective study that involved 13 Asian countries and 62 investigators.
Liver Histology
Histological changes in the liver have not been reported except in patients who had autopsies, and these showed non-specific changes or severe necrosis which could be related to hypoxic injury [53]. An autopsy study from Italy of 48 patients with COVID-19 with no prior history of liver disease or evidence of acute liver failure during hospitalization found evidence of diffuse alterations of intra-hepatic blood vessels characterized by partial or complete luminal thrombosis of portal and sinusoidal vessels and fibrosis of the portal tract [54]. Kupffer cells showed evidence of necrotic debris. Some degrees of liver enzyme alterations (AST, ALT or GGT) were seen in all but one patient and 96% had elevated levels of D-dimer. Based on this, it was tempting to speculate that coagulopathy may also play a role for abnormal liver enzymes seen in some of these patients. However, an autopsy series in 12 patients reported from the USA did not confirm these observations and showed mild portal plasma lymphocytic infiltrations and signs of fibrosis [55].
Impact of Liver Disease on Morbidity and Mortality
Overall Mortality with COVID-19
Overall mortality in COVID-19 is variable depending on geographical location, age, and comorbidities. In one study of 44,672 patients (87% between of 30 and 79 years old), 5% became critically ill with a case-fatality rate of 2.3% [56]. European countries have reported a higher mortality rate (10–16%) where as in the USA, this has been around 5.5% [57]. As of July 8, 2020, the WHO reported that worldwide mortality secondary to COVID-19 was 4.61% [58]. This high mortality could be related to a bias in reporting and testing. There is a very high mortality, ranging from 30 to 65%, among patients who requires mechanical ventilation [59, 60]. Mortality rates are higher in over people (above 70 years) and among those with preexisting comorbid conditions such as cardiovascular disease, diabetes, hypertension, cancer, obesity, and chronic respiratory diseases. Other vulnerable patient population includes those with cirrhosis and portal hypertension, HIV with low CD4 counts, immunosuppressive therapy, and liver and other solid organ transplant recipients.
Mortality in Those with Chronic Liver Disease (CLD)
There is increasing evidence that indicates a higher risk of mortality in patients with CLD who are infected with SARS -CoV-2 [60,61,62]. A large study from England that analyzed 5683 COVID-19 deaths found that 111 patients had underlying CLD, and this comorbidity was associated with an increased risk of death [adjusted OR 1.62, 95% CI 1.33–1.95] [61]. Another study that consisted of 152 patients (103 patients with cirrhosis and 49 with non-cirrhotic CLD) with COVID-19 from two international liver registries reported a very high mortality of 40% in cirrhosis and 12% in CLD without cirrhosis [62]. Moreover, 95% of cirrhotics were hospitalized, including 23% in the ICU, and 17.5% required mechanical ventilation. MELD score and Child–Pugh score had strong correlations with mortality. Mortality in patients with Child–Pugh A was 24%, while this was 63% in those with Child–Pugh C. The authors found that 37% of cirrhotics with COVID-19 had hepatic decompensation, the most frequent being worsening ascites (7%) and hepatic encephalopathy (16.5%). Variceal hemorrhage was seen only in 1%. While the majority of cirrhotics died from pneumonia (79%), liver-related death was seen only in 12%. An electronic medical record (EMR)-based study, after propensity matching, had also recently reported higher mortality in 250 patients with CLD [63]. There are few studies that had shown that patients with liver disease have no increased mortality. A recent study from the Lombardy region of Italy in 1591 patients admitted to the ICU showed that only 3% had preexisting chronic liver disease; the authors also did not report a higher prevalence of inflammatory bowel disease [47]. Nevertheless, based on majority of studies, it is reasonable to conclude that patients with underlying cirrhosis are likely to have a higher mortality with COVID-19.
There is also evidence that indicates liver enzyme abnormalities maybe a predictor of disease severity as well as mortality in COVID-19 patients. Although elevation in AST and ALT levels is the most frequently seen biochemical changes, there are also reports of elevated alkaline phosphatase, bilirubin, GGT (gamma glutamyl transpeptidase), as well as cases of decreased albumin levels in patients with severe COVID-19 infection [64,65,66]. In one large study, liver injury at admission was an independent predictor of the composite outcome of ICU admission or death (OR = 2.53, p < 0.001) [64]. A meta-analysis of seven studies that consisted of 1370 COVID-19 patients found a significant association between elevated AST levels and increased risk of mortality [standard mean deviation (SMD) = 0.75, 95% CI 0.33–1.17, p < 0.001] [65]. Similarly, increased ALT levels were also associated with increased mortality risk (SMD = 0.35, 95% CI 0.13–0.57, p = 0.002).
GI and Liver Disease-Specific Recommendations
Inflammatory Bowel Disease
Patients with active inflammatory bowel disease (IBD) are likely to have a higher risk of infection, especially if they are on immunosuppressive medication. Avoiding NSAID use would be preferable due to its association with precipitating IBD flares, and moreover, there are unconfirmed reports of more serious illness in COVID-19 patients taking NSAIDs. The impact of immunosuppression on the severity of COVID-19 disease remains unclear; nevertheless, one has to assume that they are susceptible to develop the infection and likely to have a more severe disease. The American Gastroenterology Association (AGA) recommended that IBD patients who are negative for SARS CoV-2 should continue their current IBD regimens including infusions so as to avoid relapse [67]. Those who are positive for COVID-19 may continue amino salicylates, topical rectal therapy, and oral budesonide as they are considered safe, but should temporarily discontinue thiopurines, methotrexate, tofacitinib, as well as biological agents. Combination of immunosuppressive and biologic therapy may have a higher risk than monotherapy, but currently there is no objective evidence for this. There is a concern that patients on higher dose of steroids may not do well and it is advisable to taper dose to below 20 mg if possible. Although the role of corticosteroids in the management of COVID-19 was initially questionable, recent evidence suggests low dose dexamethasone may improve mortality in patients with moderate to severe illness [68]. Initiation of immunomodulator therapy is not recommended. This recommendation is based on common sense and not on objective data. It may be prudent to avoid initiating dual therapy with biologics and immunosuppressive therapy during the pandemic.
IBD patients, like everybody else with other comorbidities, should take all precautions to avoid getting infection. IBD cancer surveillance procedures should be postponed, and IBD disease assessment endoscopy should be done preferably with a combination of symptoms assessment, biomarkers, radiology, or capsule endoscopy. Urgent management of perianal sepsis should be undertaken as an outpatient procedure, and complex IBD surgery should be deferred where possible. The exception to this rule may for subtotal colectomy in acute severe ulcerative colitis and intestinal resection to control penetrating complications of Crohn’s Disease.
GI Endoscopy
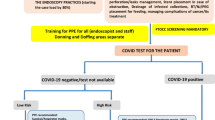
If an endoscopic procedure is deemed necessary, patients should be screened for COVID-19 if possible [69,70,71,72,73]. Ideally, all patients should be tested for SAR-CoV-2 in addition to a screening questionnaire. Unless necessary, visitors including relatives of patients should not be allowed in the endoscopy unit including reception and recovery areas. Appropriate personal protective equipment (PPE) such as gloves, mask, eye shield/goggles, face shields, and gown should be available and worn by all members of the endoscopy team. Reports from Italy suggest that infection rates among healthcare workers are as high as 20%, emphasizing the need for strict adherence to infection control precautions [74]. Additional precautions should be taken for procedures done on patients with COVID-19 positive patients or those who are suspected of COVID-19. These procedures should be done in negative pressure endoscopy rooms, and if negative pressure rooms are not available, it should be in the negative pressure ICU rooms or the operating room. Endoscopes after use should undergo standard manual cleaning followed by high-level disinfection (HLD) which refers to the use of FDA cleared chemical germicides capable of destroying all microorganisms (virus, fungi, mycobacterium, and vegetative bacteria) with the exception of some bacterial spores [75,76,77]. At present, the ASGE and ACG consider this technique adequate enough for disinfection and reprocessing of endoscopes following any procedure in confirmed or suspected patients with SAR-CoV-2.
Chronic Liver Disease Including Patients Awaiting Liver Transplant
Currently, we do not have enough data to know if patients with mild chronic liver diseases from HBV, HCV, or fatty liver disease are at increased risk to develop more severe disease. However, patients with advanced cirrhosis and liver transplant recipients should be managed as high-risk patients. The decision to perform endoscopic or radiological procedures should be made based on local infection rates.
If infection rates are high, all routine screening endoscopic procedures should be avoided. Prophylactic endoscopic banding should be discouraged. ERCP and percutaneous biliary procedures, liver biopsy, TIPS, or local ablative treatment for HCC should be done only when it is critical for patient management. Surveillance radiological procedures should be minimized but should not be delayed more than 6–8 weeks. We believe, however, that those with suspected autoimmune hepatitis should not be treated empirically without a histological confirmation since it is almost always a lifelong treatment. If the infection rates are low, the above procedures could be done with necessary precautions.
We believe that liver transplant (LT) evaluation should be reserved for patients with HCC or those with a high MELD score who are likely to benefit from immediate liver transplant listing. Laboratory and imaging studies should be ordered only if clinically necessary and not for simply updating MELD scores. However, these decisions should be modified based on local infection and hospitalization rates.
There are reports that many centers are selective in doing liver transplantation because of shortage of blood, ICU room, medical, nursing, and technical staff. A national survey of 111 solid organ transplant centers from the USA between March 24, 2020, and March 31, 2020, found that 68% of the live donor liver transplant programs had been completely suspended while only 27% of the deceased donor liver transplants were functioning without any restriction [78]. Centers were transplanting only those candidates who had high 1–3-month mortality probability, those with acute liver failure, MELD score > 25 or patients with HCC who had exhausted other treatment options. However, in areas where infection rates have come down, transplant centers have started performing liver transplant without many restrictions. Recent AASLD guidelines recommend that liver transplantation is considered lifesaving (Tier 3b on the CMS list) and should be continued [79].
If organ is available and an institutional decision was made to proceed, both recipient and donor should be screened for COVID-19. Obtaining samples from multiple sites (bronchoalveolar lavage, nasal, and pharyngeal swabs) to increase sensitivity and reduce false-negative results is encouraged. A chest radiograph may be considered in addition to the above screening techniques. Patients should be brought to the hospital only when there is a high probability that liver transplantation will proceed. Those with COVID-19 should wait at least 14–21 days after resolution of symptoms and have documented one or two negative SARS-CoV-2 PCR tests before they are considered for liver transplantation [78].
Post-transplant Patients
The effects of COVID-19 in liver transplant recipients are still unclear although early data suggested the innate immune response may be the main driver for pulmonary injury due to COVID-19. Post-transplant immunosuppression was not a risk factor for mortality with SARS in 2003 and MERS in 2012 [80]. As in IBD patients on immunosuppression, it is prudent not to alter or discontinue immunosuppressive medication doses in liver transplant recipients who do not have COVID-19 since this can precipitate an acute rejection and may require higher immunosuppression and complications.
If a transplant recipient with suspected COVID-19 needs to be hospitalized, it is preferable to admit them to non-transplant units to avoid contact with patients who had recent organ transplants. AASLD recommends discontinuing or reducing the dose of anti-metabolites (azathioprine or mycophenolate) in COVID-19 positive recipients [79]. Calcineurin inhibitors (tacrolimus) should be maintained in patients who were transplanted within the last 6 months, but the dose may be reduced in those who were transplanted more than 6 months ago. Recipients with suspected graft rejection should be started on immunosuppressive therapy irrespective of their COVID 19 status. Isolated case reports from China have described COVID-19 in liver transplant recipients with successful outcomes [81]. To date, the largest study (n = 103) in LT recipients with COVID-19 comes from the European Liver Transplant Registry (ELTR), which reported a mortality rate of 16% [82]. Of the 103 patients, 83 patients were hospitalized including 15 in the ICU. The majority of them were treated with hydroxychloroquine (66%), 18% received high-dose steroids, and 7% received tocilizumab. Unfortunately, information on immunosuppressive regimen was not available. Patients who succumbed to COVID-19 were greater than 60 years of age and were more likely to be male (88%). They also observed that recipients who were transplanted more than 2 years ago had higher odds of dying compared to those who were transplanted within the past 2 years, although this was not statistically significant. However, contrary to this study another registry analysis from Europe that consisted of 39 LT recipients with COVID-19 (9 deaths) found that at least four patients who died were transplanted within the past 2 years and had a median age less than 65 years [83]. A case series from a transplant center in Italy reported six deaths among six LT recipients who were infected with SARS-CoV-2. All three were male, older than 65 years and developed ARDS requiring mechanical ventilation [84]. The authors observed that all deaths occurred in patients transplanted > 10 years ago, while recent LT recipients (< 2 years since transplant) who were infected with SARS COV2 had a fairly uneventful course. Although no firm conclusions can be made, based on these preliminary observations one can speculate that high dose immune suppression among early LT recipients may have a protective role in preventing the heightened immune response that is frequently seen in COVID 19 patients who develop severe disease.
Conclusion
As the COVID-19 pandemic continues to grow worldwide, our knowledge regarding its epidemiology, modes of transmission, and protean clinical presentations continues to evolve. We understand that gastrointestinal complaints and liver enzyme abnormalities are frequently seen in patients with COVID-19, especially in those with severe disease. Patients who present atypically with only gastrointestinal symptoms may also be at risk of delayed diagnosis and possibly more severe disease. Fecal–oral transmission is possible, but it is not clearly documented. The causes of liver enzyme abnormalities are multifactorial, but there is no increased risk of infection in those with underlying liver disease or liver transplant recipients. However, those with CLD are more likely to have a higher mortality if they were to develop COVID-19.
References
Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269.
Day M. Covid-19: identifying and isolating asymptomatic people helped eliminate virus in Italian village. BMJ. 2020;368:m1165. https://doi.org/10.1136/bmj.m1165.
Nishiura H, Kobayashi T, Miyama T, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int J Infect Dis. 2020;94:154–155.
https://news.usc.edu/170565/covid-19-antibody-study-coronavirus-infections-los-angeles-county/.
Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020. https://doi.org/10.1016/S0140-6736(20)31304-0.
Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020. https://doi.org/10.7326/M20-3012.
Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun.. 2020;525:135–140.
Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271.e8–280.e8. https://doi.org/10.1016/j.cell.2020.02.052.
Burgueño JF, Reich A, Hazime H, et al. Expression of SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in the gut of patients with IBD. Inflamm Bowel Dis. 2020;26:797–808. https://doi.org/10.1093/ibd/izaa085.
Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. https://doi.org/10.1007/s00405-020-05965-1.
Luo S, Zhang X, Xu H. Don’t overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19). Clin Gastroenterol Hepatol. 2020;18:1636–1637.
Wei-jie Guan, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720.
Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
Pan L, Mu M, Ren HG, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773.
Han C, Duan C, Zhang S, et al. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020;115:916–923.
Cheung KS, Hung IF, Chan PP, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020. https://doi.org/10.1053/j.gastro.2020.03.065.
Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069.
Jin X, Lian J, Hu J, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009.
Cholankeril G, Podboy A, Aivaliotis VI, et al. High prevalence of concurrent gastrointestinal manifestations in patients with SARS-CoV-2: early experience from California. Gastroenterology. 2020. https://doi.org/10.1053/j.gastro.2020.04.008.
Chen A, Agarwal A, Ravindran N, To C, Zhang T, Thuluvath PJ. Are gastrointestinal symptoms specific for COVID-19 infection? A prospective case-control study from the United States. Gastroenterology. 2020. https://doi.org/10.1053/j.gastro.2020.05.036.
Nobel YR, Phipps M, Zucker J, et al. Gastrointestinal symptoms and coronavirus disease 2019: a case-control study from the United States. Gastroenterology. 2020. https://doi.org/10.1053/j.gastro.2020.04.0.
Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020;382:2372–2374.
Sattar Y, Connerney M, Rauf H, et al. Three cases of COVID-19 disease with colonic manifestations. Am J Gastroenterol. 2020;115:948–950.
Guotao L, Xingpeng Z, Zhihui D, Huirui W. SARS-CoV-2 infection presenting with hematochezia. Med Mal Infect. 2020;50:293–296. https://doi.org/10.1016/j.medmal.2020.03.005.
Bezzio C, Saibeni S, Variola A, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69:1213–1217. https://doi.org/10.1136/gutjnl-2020-32141.
Xiao F, Tang M, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831.e3–1833.e3.
Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is fecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335–337.
Chen C, Gao G, Xu Y, et al. SARS-CoV-2-positive sputum and feces after conversion of pharyngeal samples in patients with COVID-19. Ann Intern Med. 2020. https://doi.org/10.7326/m20-0991.
Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in feces of COVID-19 patients. J Med Virol. 2020;92:833–840.
Chen L, Lou J, Bai Y, Wang M. COVID-19 disease with positive fecal and negative pharyngeal and sputum viral tests. Am J Gastroenterol. 2020. https://doi.org/10.14309/ajg.0000000000000610.
Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494.
Ling Y, Xu SB, Lin YX, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl). 2020;133:1039–1043.
Hadi A, Werge M, Kristiansen KT, et al. Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: case report on three family members. Pancreatology. 2020;20:665–667.
Aloysius MM, Thatti A, Gupta A, Sharma N, Bansal P, Goyal H. COVID-19 presenting as acute pancreatitis. Pancreatology.. 2020. https://doi.org/10.1016/j.pan.2020.05.003.
Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q. Pancreatic injury patterns in patients with COVID-19 pneumonia. Gastroenterology. 2020;159:367–370.
Asti E, Lovece A, Bonavina L. Gangrenous cholecystitis during hospitalization for SARS-CoV2 infection. Updates Surg.. 2020. https://doi.org/10.1007/s13304-020-00814-6.
Singh R, Domenico C, Rao SD, et al. Novel coronavirus disease 2019 in a patient on durable left ventricular assist device support. J Card Fail. 2020;26:438–439. https://doi.org/10.1016/j.cardfail.2020.04.007.
Shi H, Han X, Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434.
Kovalic A, Huang G, Thuluvath PJ, Satapathy SK. Elevated liver biochemistries in hospitalized Chinese patients with severe COVID-19: systematic review and meta-analysis. Hepatology. 2020. https://doi.org/10.1002/hep.31472.
Kunutsor SK, Laukkanen JA. Markers of liver injury and clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. J Infect. 2020. https://doi.org/10.1016/j.jinf.2020.05.045.
Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19-preliminary report. N Engl J Med.. 2020. https://doi.org/10.1056/nejmoa2007764.
Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med.. 2020. https://doi.org/10.1056/nejmoa2015301.
Cai Q, Huang D, Yu H, et al. COVID-19: abnormal liver function tests. J Hepatol. 2020. https://doi.org/10.1016/j.jhep.2020.04.006.
Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and liver. J Hepatol. 2020. https://doi.org/10.1016/j.jhep.2020.06.006.
Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. https://doi.org/10.1001/jama.2020.5394.
Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: a meta-analysis. Liver Int. 2020;40:1316–1320.
Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020. https://doi.org/10.1007/s12072-020-10078-2.
Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513.
Qiu H, Wander P, Bernstein D, Satapathy SK. Acute on chronic liver failure from novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Liver Int. 2020. https://doi.org/10.1111/liv.14506.
Sarin SK, Choudhury A, Lau GK, et al. Pre-existing liver disease is associated with poor outcomes with SARS CoV2 infection: the APCOLIS study (APASL COVID-19 liver injury spectrum study). Hepatology Int. 2020;4:1–11. https://doi.org/10.1007/s12072-020-10072-8.
Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422.
Sonzogni A, Previtali G, Seghezzi M, Alessio MG, Gianatti A, Licini L, et al. Liver and COVID 19 infection: a very preliminary lesson learnt from histological post-mortem findings in 48 patients. 2020. https://doi.org/10.20944/preprints202004.0438.v1.
Schaller T, Hirschbühl K, Burkhardt K, et al. Postmortem examination of patients with COVID-19. JAMA. 2020;323:2518–2520.
The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 Novel Coronavirus Diseases (COVID-19), China, 2020. China CDC Wkly. 2020;2:113–122.
Bhatraju PK, Ghassemieh BJ, Nichols M, et al. COVID-19 in critically ill patients in the seattle region: case series. N Engl J Med. 2020;382:2012–2022.
Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:e26.
Williamson E, Bhaskaran KJ, Bacon S, et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. MedRxiv. 2020. https://doi.org/10.1101/2020.05.06.20092999.
Moon AM, Webb GJ, Aloman C, et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020. https://doi.org/10.1016/j.jhep.2020.05.013.
Singh S, Khan A. Clinical characteristics and outcomes of COVID-19 among patients with pre-existing liver disease in United States: A multi-center research network study. Gastroenterology. 2020. https://doi.org/10.1053/j.gastro.2020.04.064.
Hajifathalian K, Krisko T, Mehta A. Gastrointestinal and hepatic manifestations of 2019 novel Coronavirus disease in a large cohort of infected patients from New York: clinical implications for prognosis. Gastroenterology. 2020. https://doi.org/10.1053/j.gastro.2020.05.010.
Wang Y, Shi L, Wang Y, Yang H. An updated meta-analysis of AST and ALT levels and the mortality of COVID-19 patients. Am J Emerg Med. 2020. https://doi.org/10.1016/j.ajem.2020.05.063.
Aziz M, Fatima R, Lee-Smith W, Assaly R. The association of low serum albumin level with severe COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24:255. https://doi.org/10.1186/s13054-020-02995-3.
Rubin DT, Feuerstein JD, Wang AY, Cohen RD. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology. 2020;159:350–357.
Mahase E. COVID-19: low dose steroid cuts death in ventilated patients by one third, trial finds. BMJ. 2020;369:m2422. https://doi.org/10.1136/bmj.m2422.
Repici A, Maselli R, Colombo M, et al. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020;92:192–197.
https://gi.org/2020/03/15/joint-gi-society-message-on-covid-19/.
Boettler T, Newsome PN, Mondelli MU, et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep.. 2020;2:100113. https://doi.org/10.1016/j.jhepr.2020.100113.
Remuzzi A, Remuzzi G. COVID-19 and Italy: What next? Lancet. 2020;395:1225–1228.
Nelson DB. Cleaning and disinfecting gastrointestinal endoscopic equipment. Clin Gastrointest Endosc. 2012. https://doi.org/10.1016/b978-1-4377-1529-3.00004-x.
Standards of Infection Prevention in Reprocessing of Flexible Gastrointestinal Endoscopes. Society of Gastroenterology Nurses and Associates (SGNA) Practice Committee, 2017–2018. https://www.sgna.org/Portals/0/SGNA/Standardsofinfectionpreventioninreprocessing_FINAL.pdf?ver=2018-11-16-084835-387. Accessed June 15 2020.
Boyarsky BJ, Po-Yu Chiang T, Werbel WA, et al. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant.. 2020;20:1809–1818.
Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatology. 2020. https://doi.org/10.1002/hep.31281.
D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020. https://doi.org/10.1002/lt.25840.
Liu B, Wang Y, Zhao Y, et al. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am J Transplant. 2020;20:1891–1895.
Belli LS, Duvoux C, Karam V, et al. COVID-19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol. 2020. https://doi.org/10.1016/s2468-1253(20)30183-7.
Webb GJ, Moon AM, Barnes E, Barritt AS, Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;5:643–644. https://doi.org/10.1016/S2468-1253(20)30125-4.
Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant center in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532–533.
Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–2059.
Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan. China. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa270.
Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. https://doi.org/10.1136/gutjnl-2020-3.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Informed consent
Review article and not applicable. The study has not been published or submitted elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thuluvath, P.J., Alukal, J.J., Ravindran, N. et al. What GI Physicians Need to Know During COVID-19 Pandemic. Dig Dis Sci 66, 2865–2875 (2021). https://doi.org/10.1007/s10620-020-06625-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06625-4