Summary
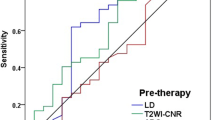
Objectives To determine the quantitative parameters of DCE-US for predicting early functional response of patients with metastatic gastrointestinal stromal tumors (GIST). Materials and methods Phase II multicentre clinical trial in patients with metastatic GIST treated with masatinib mesylate (7.5 mg/kg daily by oral route) Patients followed using three different imaging techniques: 1) DCE-US before treatment and on days 1, 7, 15 and after 1, 2, 4, 6 months and every 3 months. 2) CT assessments, using RECIST criteria, before treatment, after 2, 4, 6 months and then every 3 months. 3) FDG PET before treatment and after 1 month. Results Twenty patients included and followed-up for up to 36 months, with 269 DCE-US examinations performed. No significant changes in the 7 selected DCE-US variables on day 1 and 7 vs baseline. On day 15, significant reductions in all the variables related to blood volume recorded: area under the curve (AUC) (p = 0. 004), area under the wash-in (AUWI) (p = 0.002), area under the wash-out (AUWO) (p = 0.002) and Peak Intensity (p = 0.005). Also slope of wash-in changed significantly (p = 0.003). An important reduction in Standard Uptake Values (SUV) recorded in 7/11 patients (PFS >18 months). Decrease in DCE-US AUC, AUWI and AUWO values on day 7 were predictive of PET-CT results. Conclusions AUC AUWI, AUWO are the DCE-US parameters related to blood volume that at D 15 can predict the response of GISTs to treatment with masatinib. Additional studies are ongoing.





Similar content being viewed by others
References
Connolly EM, Gaffney E, Reynolds JV (2003) Gastrointestinal stromal tumours. Br J Surg 90:1178–1186. doi:10.1002/bjs.4352
Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M (1998) CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol 11:728–734
Bono P, Krause A, Von Mehren M et al (2004) Serum KIT and KIT ligand levels in patients with gastrointestinal stromal tumors treated with imatinib. Blood 103:2929–2935. doi:10.1182/blood-2003-10-3443
Rubin BP, Singer S, Tsao C et al (2001) KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res 61:8118–8121
Zhu J, Wang Y, Hou M, Li HY, Zhang J (2007) Imatinib mesylate treatment for advanced gastrointestinal stromal tumor: a pilot study focusing on patients experiencing sole liver metastasis after a prior radical resection. Oncology 73:324–327. doi:10.1159/000134475
Joensuu H, De Braud F, Coco P et al (2008) Phase II open-label study of PTK787/ZK222684 for the treatment of metastatic gastrointestinal stromal tumors resistant to imatinib mesylate. Ann Oncol 19:173–177. doi:10.1093/annonc/mdm419
Nishida T, Shirao K, Sawaki A et al (2008) Efficacy and safety profile of imatinib mesylate (ST1571) in Japanese patients with advanced gastrointestinal stromal tumors: a phase II study (STi571B1202). Int J Clin Oncol 13:244–251. doi:10.1007/s10147-007-0746-y
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Van der Abbeele AD, for the GIST PET study group (2001) F18-FDG-PET provides early evidence of biological response to STI571 in patients with malignant gastrointestinal stromal tumors (GIST). Proc Am Soc Clin Oncol 20:362a
Choi H, Charnsangavej C, Faria SC et al (2007) Correlation of Computed Tomography and Positron Emission Tomography of patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 25:1753–1759. doi:10.1200/JCO.2006.07.3049
Van den Abeele AD (2008) The lesions of GIST-PET and PET-CT: a new paradigm for imaging. Oncologist 13(Suppl 2):8–13. doi:10.1634/theoncologist.13-S2-8
Lassau N, Chami L, Benatsou B et al (2007) Dynamic contrast-enhanced ultrasonography (DCE-US) with quantification of tumor perfusion: a new diagnostic tool to evaluate the early effects of antiangiogenic treatment. Eur Radiol 17(Suppl 6):F89–F98
Italiano A, Bui B (2008) Gastrointestinal stromal tumors: molecular aspects and therapeutic implications. Bull Cancer 95:107–116. doi:10.1684/bdc.2008.0561
Le Cesne A, Blay JY, Bui BN, Bouché O, Adenis A, Domont J, Cioffi A, Ray-Coquard I, Lassau N, Bonvalot S, Moussy A, Kinet JP, Hermine O (2010) Phase II study of oral masitinib mesilate in imatinib-naïve patients with locally advanced or metastatic gastro-intestinal stromal tumour (GIST). Eur J Cancer 46(8):1344–1351. doi:10.1016/j.ejca.2010.02.014
Fletcher CD, Berman JJ, Corless C et al (2002) Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol 10:81–89
Lassau N, Lamuraglia M, Chami L et al (2006) Gastrointestinal stromal tumors treated with imatinib: monitoring response with contrast-enhanced sonography. AJR Am J Roentgenol 187(5):1267–1273. doi:10.2214/AJR.05.1192
Antoch G, Kanja J, Bauer S et al (2004) Comparison of PET, CT and dual-modality PET/CT imaging for monitoring of imatinib (ST1571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med 45:357–365
Bauer A, Solbiati L, Weissman N (2002) Ultrasound imaging with SonoVue: low mechanical index real-time imaging. Acad Radiol 9(suppl 2):S282–S284
Peronneau P, Lassau N, Leguerney I, Cosgrove D (2010) Contrast ultrasonography: necessity of using linear data processing for quantification of tumor vascularisation. Ultraschall Med 31(4):370–378. doi:10.1055/s-0029-1245450
Lassau N, Chebil M, Chami L, Bidault S, Girad E, Roche A (2010) Dynamic contrast-enhanced ultrasonography (DCE-US): a new tool for the early evaluation of antiangiogenic treatment. Targeted Oncol 5(1):53–58. doi:10.1007/s11523-010-0136-7
Lassau N, Chami L, Benatsou B, Peronneau P, Roche A (2007) Dynamic contrast-enhanced ultrasonography (DCE-US) with quantification of tumor perfusion: a new diagnostic tool to evaluate the early effects of antiangiogenic treatment. Eur Radiol 17(Suppl 6):F89–F98
Lassau N, Brule A, Chami L, Benatsou B, Péronneau P, Roche A (2008) Evaluation of early response to antiangiogenic treatment with dynamic contrast enhanced ultrasound. J Radiol 89(5 Pt 1):549–555, doi:JR-5-2008-89-5-C1-0221-0363-101019-200802781
Lassau N, Koscielny S, Albiges L, Chami L, Benatsou B, Chebil M, Roche A, Escudier BJ (2010) Metastatic renal cell carcinoma treated with sunitinib: early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin Cancer Res 15; 16(4):1216–1225. doi:10.1158/1078-0432.CCR-09-2175
Lamuraglia M, Le Cesne A, Chami L, et al (2006) Dynamic contrast-enhanced Doppler ultrasound (DCE-US) is a useful radiological assessment to early predict the outcome of patients with gastrointestinal stromal tumors (GIST) treated with imatinib (IM). J Clin Oncol ASCO Annual Meeting Proceedings (Post Meeting Edition) June 20, Vol 24:529S
Lamuraglia M, Escudier B, Chami L et al (2006) To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: pilot study using dynamic contrast enhanced Doppler ultrasound. Eur J Cancer 42(15):2472–2479. doi:10.1016/j.ejca.2006.04.023
Disclosure
Disclosure of funding received from any of the following organizations: NIH—Wellcome Trust, Howard Hughes Medical Institute and others.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lassau, N., Chami, L., Koscielny, S. et al. Quantitative functional imaging by Dynamic Contrast Enhanced Ultrasonography (DCE-US) in GIST patients treated with masatinib. Invest New Drugs 30, 765–771 (2012). https://doi.org/10.1007/s10637-010-9592-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9592-2