Summary
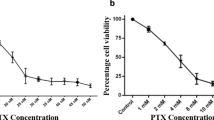
Background Paclitaxel is an effective antimitotic agent in cancer treatment; however, one of its most common toxicities is hypersensitivity due to excipients used for water solubility. Nanoparticulate paclitaxel (Crititax®, CTI52010) is paclitaxel that consists only of nanoparticulate drug in saline. Our objective was to examine the effect of nanoparticulate paclitaxel on prostate cancer cells derived from castration-resistant prostate cancer in men and dogs, as companion dogs represent a unique naturally occurring model of castration-resistant prostate cancer. We hypothesized that nanoparticulate paclitaxel would be effective in affecting cell viability, colony forming ability, apoptosis, and induction of structural changes to the microtubules of prostate cancer cells. Methods Human PC3 and canine Ace-1 cells were treated with 0.001–1.0 μm concentrations of paclitaxel and nanoparticulate paclitaxel. Cell viability, apoptosis, and colony forming assays were analyzed and compared in the presence of both drugs. Microtubule structure was examined by fluorescence microscopy following incubation with drug. Results Nanoparticulate paclitaxel was as effective as standard paclitaxel in decreasing cell viability, decreasing colony forming ability, and inducing apoptosis in human and canine prostate cancer cells in a dose-dependent manner. Fluorescence microscopy confirmed the microtubule target of nanoparticulate paclitaxel. Conclusions Nanoparticulate paclitaxel is as effective as paclitaxel in decreasing cell viability, initiating apoptosis, decreasing cell survival, and causing rigidity of microtubules in both human and canine castration-resistant prostate cancer. This represents an attractive area for further study, using the companion dog as a model for disease in men.





Similar content being viewed by others
References
Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277:665–667
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT (1971) Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 93:2325–2327
Singla AK, Garg A, Aggarwal D (2002) Paclitaxel and its formulations. Int J Pharm 235:179–192
Zhao L, Ye Y, Li J, Wei YM (2011) Preparation and the in-vivo evaluation of paclitaxel liposomes for lung targeting delivery in dogs. J Pharm Pharmacol 63:80–86
Yeh TK, Lu Z, Wientjes MG, Au JL (2005) Formulating paclitaxel in nanoparticles alters its disposition. Pharm Res 22:867–874
Tanaka T, Decuzzi P, Cristofanilli M, Sakamoto JH, Tasciotti E et al (2009) Nanotechnology for breast cancer therapy. Biomed Microdevices 11:49–63
Bulitta JB, Zhao P, Arnold RD, Kessler DR, Daifuku R et al (2009) Mechanistic population pharmacokinetics of total and unbound paclitaxel for a new nanodroplet formulation versus Taxol in cancer patients. Cancer Chemother Pharmacol 63:1049–1063
Miele E, Spinelli GP, Tomao F, Tomao S (2009) Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int J Nanomedicine 4:99–105
Roby KF, Niu F, Rajewski RA, Decedue C, Subramaniam B et al (2008) Syngeneic mouse model of epithelial ovarian cancer: effects of nanoparticulate paclitaxel, Nanotax. Adv Exp Med Biol 622:169–181
Axiak SM, Selting KA, Decedue CJ, Henry CJ, Tate D et al (2011) Phase I dose escalation safety study of nanoparticulate paclitaxel (CTI 52010) in normal dogs. Int J Nanomedicine 6:2205–2212
Niu F, Roby KF, Rajewski RA, Decedue C, Subramaniam (2006) Paclitaxel nanoparticles: production using compressed CO2 as antisolvent, characterization and animal model studies. In: Svenson S (ed) Polymeric drug delivery volume II- polymeric matrices and drug particle engineering. ACS Symposium Series, vol. 924. American Chemical Society, Washington, DC.
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
Mancuso A, Oudard S, Sternberg CN (2007) Effective chemotherapy for hormone-refractory prostate cancer (HRPC): present status and perspectives with taxane-based treatments. Crit Rev Oncol Hematol 61:176–185
Leroy BE, Northrup N (2009) Prostate cancer in dogs: comparative and clinical aspects. Vet J 180:149–162
Paoloni M, Khanna C (2008) Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer 8:147–156
Bryan JN, Keeler MR, Henry CJ, Bryan ME, Hahn AW et al (2007) A population study of neutering status as a risk factor for canine prostate cancer. Prostate 67:1174–1181
Khanna C, London C, Vail D, Mazcko C, Hirschfeld S (2009) Guiding the optimal translation of new cancer treatments from canine to human cancer patients. Clin Cancer Res 15:5671–5677
Vail DM, MacEwen EG (2000) Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest 18:781–792
LeRoy BE, Thudi NK, Nadella MV, Toribio RE, Tannehill-Gregg SH et al (2006) New bone formation and osteolysis by a metastatic, highly invasive canine prostate carcinoma xenograft. Prostate 66:1213–1222
Tai S, Sun Y, Squires JM, Zhang H, Oh WK et al (2011) PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 71:1668–1679
Keller JM, Schade GR, Ives K, Cheng X, Rosol TJ, et al (2013) A novel canine model for prostate cancer. Prostate 743:952–959
Thudi NK, Martin CK, Nadella MV, Fernandez SA, Werbeck JL et al (2008) Zoledronic acid decreased osteolysis but not bone metastasis in a nude mouse model of canine prostate cancer with mixed bone lesions. Prostate 68:1116–1125
Thudi NK, Martin CK, Murahari S, Shu ST, Lanigan LG et al (2011) Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate 71:615–625
Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N et al (2005) Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 23:7794–7803
Sella A, Konichezky M, Flex D, Sulkes A, Baniel J (2000) Low PSA metastatic androgen- independent prostate cancer. Eur Urol 38:250–254
Acknowledgments
Nanoparticulate paclitaxel (CTI52010, Crititax®), was provided by CritiTech, Inc.
Disclosures
CD is Chief Scientific Officer and JE is Director of Laboratory Operations for CritiTech, Inc., manufacturer of Crititax®.
Grant support
This work was supported by a University of Missouri Phi Zeta grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Work performed at The Comparative Oncology and Epigenetics Laboratory, Department of Veterinary Medicine and Surgery, University of Missouri
Rights and permissions
About this article
Cite this article
Axiak-Bechtel, S.M., Kumar, S.R., Dank, K.K. et al. Nanoparticulate paclitaxel demonstrates antitumor activity in PC3 and Ace-1 aggressive prostate cancer cell lines. Invest New Drugs 31, 1609–1615 (2013). https://doi.org/10.1007/s10637-013-0006-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-0006-0